Key Points
-
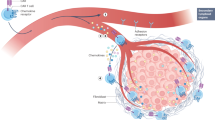
Graft-versus-leukaemic effects improve survival following allogeneic stem cell transplantation for childhood leukaemia, and provide proof-of-principle that immune mediated effects can eradicate chemoresistant cancer cells
-
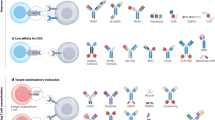
Anti-GD2 monoclonal antibodies can be curative when administered in the setting of minimal residual disease for neuroblastoma, and efficacy requires optimal induction of antibody-dependent cell-mediated cytotoxicity (ADCC)
-
In general, tumour vaccines do not regress established tumours, but can diminish tumour growth or recurrence; randomized studies will be needed to define their efficacy in paediatric cancer
-
Adoptive T-cell immunotherapies have demonstrated efficacy against Epstein–Barr virus-associated lymphoproliferative neoplasms and impressive results have been reported using genetically engineered T cells to treat paediatric leukemia
-
Expansion of the immunotherapeutic armamentarium for paediatric cancers hinges on identification of new immune targets
-
Toxicity associated with immunotherapies is distinct from toxicity associated with cytotoxic agents and small-molecule kinase inhibitors; most common toxic effects include cytokine-release syndrome and autoimmune reactions
Abstract
After decades of research, immunotherapies for cancer are demonstrating increasing success. These agents can amplify existent antitumour immunity or induce durable antitumour immune responses in a wide array of cancers. The spectrum of immunotherapeutics is broad, spanning monoclonal antibodies and their derivatives, tumour vaccines, and adoptive therapies using T cells and natural killer cells. Only a small number of immunotherapies have been tested in paediatric cancers, but impressive antitumour effects have already been observed. Mononclonal antibodies targeting GD2 that induce antibody-dependent cell-mediated cytotoxicity improve survival in high-risk neuroblastoma. Bi-specific monoclonal antibodies that simultaneously target CD19 and activate T cells can induce remission in acute B-cell lymphoblastic leukaemia (B-ALL) and adoptive immunotherapy using T cells genetically engineered to express chimeric antigen receptors targeting CD19 induce impressive responses in B-ALL. Efforts are underway to generate and test new immunotherapies in a wider array of paediatric cancers. Major challenges include a need to identify immunotherapy targets on the most lethal childhood cancers, to expand availability of technology-intense platforms, such as adoptive cell therapy, to optimize management of novel toxicities associated with this new class of cancer therapies and to determine how best to incorporate these therapies into standard treatment paradigms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Smith, M. A., Altekruse, S. F., Adamson, P. C., Reaman, G. H. & Seibel, N. L. Declining childhood and adolescent cancer mortality. Cancer 120, 2497–2506 (2014).
Yu, A. L. et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 363, 1324–1334 (2010).
Grupp, S. A. et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518 (2013).
Lee, D. W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukemia in children and young adults: a phase 1 dose-escalation trial. Lancet http://dx.doi.org/10.1016/S0140-6736(14)61403-3 (2014).
Lankester, A. C. et al. Will post-transplantation cell therapies for paediatric patients become standard of care? Biol Blood Marrow Transplant http://dx.doi.org/10.1016/j.bbmt.2014.07.018 (2014).
Ruggeri, L. et al. Effectiveness of donor natural killer cell alloreactivity in mismatched haematopoietic transplants. Science 295, 2097–2100 (2002).
Bari, R. et al. Effect of donor KIR2DL1 allelic polymorphism on the outcome of paediatric allogeneic haematopoietic stem-cell transplantation. J. Clin. Oncol. 31, 3782–3790 (2013).
Leung, W. Use of NK cell activity in cure by transplant. Br. J. Haematol. 155, 14–29 (2011).
Leone, P. et al. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumour cells. J. Natl Cancer Inst. 105, 1172–1187 (2013).
Locatelli, F., Moretta, F., Brescia, L. & Merli, P. Natural killer cells in the treatment of high-risk acute leukaemia. Semin. Immunol. 26, 173–179 (2014).
Zhou, H. et al. Donor selection for killer immunoglobulin-like receptors B haplotype of the centromeric motifs can improve the outcome after HLA-identical sibling haematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 20, 98–105 (2014).
Schumm, M. et al. Depletion of T-cell receptor alpha/beta and CD19 positive cells from apheresis products with the CliniMACS device. Cytotherapy 15, 1253–1258 (2013).
Michaelis, S. U. et al. KIR haplotype B donors but not KIR-ligand mismatch result in a reduced incidence of relapse after haploidentical transplantation using reduced intensity conditioning and CD3/CD19-depleted grafts. Ann. Haematol. http://dox.doi.org/10.1007/s00277-014-2084-2 (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Miller, J. S. et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105, 3051–3057 (2005).
Rubnitz, J. E. et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J. Clin. Oncol. 28, 955–959 (2010).
Cho, D. et al. Cytotoxicity of activated natural killer cells against paediatric solid tumours. Clin. Cancer Res. 16, 3901–3909 (2010).
Zhang, H. et al. Activating signals dominate inhibitory signals in CD137L/IL-15 activated natural killer cells. J. Immunother. 34, 187–195 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Merchant, M. S. et al. Phase I trial and pharmacokinetic study of lexatumumab in paediatric patients with solid tumours. J. Clin. Oncol. 30, 4141–4147 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Alvarez-Rueda, N. et al. A monoclonal antibody to O-acetyl-GD2 ganglioside and not to GD2 shows potent anti-tumour activity without peripheral nervous system crossreactivity. PLoS ONE 6, e25220 (2011).
Cheung, N. K., Burch, L., Kushner, B. H. & Munn, D. H. Monoclonal antibody 3F8 can effect durable remissions in neuroblastoma patients refractory to chemotherapy: a phase II trial. Prog. Clin. Biol. Res. 366, 395–400 (1991).
Frost, J. D. et al. A phase I/IB trial of murine monoclonal anti-GD2 antibody 14.G2a plus interleukin-2 in children with refractory neuroblastoma: a report of the Children's Cancer Group. Cancer 80, 317–333 (1997).
Ozkaynak, M. F. et al. Phase I study of chimeric human/murine anti-ganglioside G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after haematopoietic stem-cell transplantation: a Children's Cancer Group Study. J. Clin. Oncol. 18, 4077–4085 (2000).
Simon, T. et al. Consolidation treatment with chimeric anti-GD2-antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J. Clin. Oncol. 22, 3549–3557 (2004).
Cheung, N. K. et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J. Clin. Oncol. 30, 3264–3270 (2012).
Shusterman, S. et al. Antitumour activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study. J. Clin. Oncol. 28, 4969–4975 (2010).
Delgado, D. C. et al. Genotypes of NK cell KIR receptors, their ligands, and Fcgamma receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy. Cancer Res. 70, 9554–9561 (2010).
Venstrom, J. M. et al. KIR and HLA genotypes are associated with disease progression and survival following autologous haematopoietic stem cell transplantation for high-risk neuroblastoma. Clin. Cancer Res. 15, 7330–7334 (2009).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Chao, M. P. et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142, 699–713 (2010).
Roth, M. et al. Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer 120, 548–554 (2014).
Navid, F. et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J. Clin. Oncol. 32, 1445–1452 (2014).
Barth, M., Raetz, E. & Cairo, M. S. The future role of monoclonal antibody therapy in childhood acute leukaemias. Br. J. Haematol. 159, 3–17 (2012).
Dunleavy, K. et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N. Engl. J. Med. 368, 1408–1416 (2013).
Samochatova, E. V. et al. Therapy of advanced-stage mature B-cell lymphoma and leukemia in children and adolescents with rituximab and reduced intensity induction chemotherapy (B-NHL 2004M Protocol): the results of a multicentre study. J. Paediatr. Haematol. Oncol. 36, 395–401 (2014).
Wayne, A. S. et al. Anti-CD22 immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-positive haematologic malignancies of childhood: preclinical studies and phase I clinical trial. Clin. Cancer Res. 16, 1894–1903 (2010).
Wayne, A. S., Fitzgerald, D. J., Kreitman, R. J. & Pastan, I. Immunotoxins for leukemia. Blood 123, 2470–2477 (2014).
Palanca-Wessels, M. C. & Press, O. W. Advances in the treatment of haematologic malignancies using immunoconjugates. Blood 123, 2293–2301 (2014).
Sievers, E. L. & Senter, P. D. Antibody-drug conjugates in cancer therapy. Annu. Rev. Med. 64, 15–29 (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Topp, M. S. et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J. Clin. Oncol. 29, 2493–2498 (2011).
Schlegel, P. et al. Paediatric posttransplant relapsed/refractory B-precursor acute lymphoblastic leukemia shows durable remission by therapy with the T-cell engaging bispecific antibody blinatumomab. Haematologica 99, 1212–1219 (2014).
von Stackelberg, A. et al. A Phase 1/2 Study of Blinatumomab in Paediatric Patients with Relapsed/Refractory B-Cell Precursor Acute Lymphoblastic Leukemia in American Society of Haematology [abstract], ASH Annual Meeting a70 (2013).
Teachey, D. T. et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 121, 5154–5157 (2013).
Vyas, M., Koehl, U., Hallek, M. & von Strandmann, E. P. Natural ligands and antibody-based fusion proteins: harnessing the immune system against cancer. Trends Mol. Med. 20, 72–82 (2014).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Merchant, M. S., Baird, K., Wexler, L. H., Rodriguez-Galindo, R. & Mackall, C. L. Ipilimumab: First results of a phase I trial in paediatric patients with advanced solid tumours [abstract]. J. Clin. Oncol. 30 (Suppl.), a9545 (2012).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
van der Bruggen, P. et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254, 1643–1647 (1991).
Rosenberg, S. A., Yang, J. C. & Restifo, N. P. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 10, 909–915 (2004).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Madan, R. A., Gulley, J. L., Fojo, T. & Dahut, W. L. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist 15, 969–975 (2010).
Stein, W. D. et al. Tumour regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin. Cancer Res. 17, 907–917 (2011).
Robert-Tissot, C., Nguyen, L. T., Ohashi, P. S. & Speiser, D. E. Mobilizing and evaluating anticancer T cells: pitfalls and solutions. Expert Rev. Vaccines 12, 1325–1340 (2013).
Kantoff, P. W. et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 28, 1099–1105 (2010).
Sampson, J. H. et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 28, 4722–4729 (2010).
Mackall, C. L. et al. Survival in metastatic Ewing sarcoma (EWS) and rhabdomyosarcoma (RMS) following consolidation immunotherapy with autologous lymphocyte infusion, dendritic cell vaccines ± CYT107 (rhIL-7) [abstract]. J. Clin. Oncol. 31 (Suppl.), a10013 (2013).
Scholler, J. et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 4, 132ra53 (2012).
Gattinoni, L., Powell, D. J. Jr, Rosenberg, S. A. & Restifo, N. P. Adoptive immunotherapy for cancer: building on success. Nat. Rev. Immunol. 6, 383–393 (2006).
Cui, Y. et al. Harnessing the physiology of lymphopenia to support adoptive immunotherapy in lymphoreplete hosts. Blood 114, 3831–3840 (2009).
Dudley, M. E. et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 23, 2346–2357 (2005).
Rosenberg, S. A. et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 17, 550–557 (2011).
Hong, J. J. et al. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin. Cancer Res. 16, 4892–4898 (2010).
Tran, E. et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344, 641–645 (2014).
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013).
Leen, A. M. et al. Multicentre study of banked third-party virus-specific T cells to treat severe viral infections after haematopoietic stem cell transplantation. Blood 121, 5113–5123 (2013).
Doubrovina, E. et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic haematopoietic cell transplantation. Blood 119, 2644–2656 (2012).
Icheva, V. et al. Adoptive transfer of Epstein-Barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J. Clin. Oncol. 31, 39–48 (2013).
Kapatai, G. & Murray, P. Contribution of the Epstein Barr virus to the molecular pathogenesis of Hodgkin lymphoma. J. Clin. Pathol. 60, 1342–1349 (2007).
Kuppers, R., Engert, A. & Hansmann, M. L. Hodgkin lymphoma. J. Clin. Invest. 122, 3439–3447 (2012).
Bollard, C. M. et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J. Clin. Oncol. 32, 798–808 (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Robbins, P. F. et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J. Immunol. 180, 6116–6131 (2008).
Robbins, P. F. et al. Tumour regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 29, 917–924 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Lai, J. P. et al. NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumours: significance for NY-ESO-1-based targeted therapy and differential diagnosis. Mod. Pathol. 25, 854–858 (2012).
Park, J. R. et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 15, 825–833 (2007).
Pule, M. A. et al. Virus-specific T cells engineered to coexpress tumour-specific receptors: persistence and antitumour activity in individuals with neuroblastoma. Nat. Med. 14, 1264–1270 (2008).
Louis, C. U. et al. Antitumour activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Savoldo, B. et al. CD28 co-stimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. Invest. 121, 1822–1826 (2011).
Kochenderfer, J. N. et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010).
Brentjens, R. J. et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118, 4817–4828 (2011).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011).
Kochenderfer, J. N. et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–2720 (2012).
Grupp, S. A. et al. 67 T cells engineered with a chimeric antigen receptor (CAR) targeting CD19 (CTL019) produce significant in vivo proliferation, complete responses and long-term persistence without GVHD in children and adults with relapsed, refractory ALL [abstract]. ASH Annual Meeting a67 (2013).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. http://dx.doi.org/10.1056/NEJMoa1407222 (2014).
Davila, M. L. et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6, 224ra25 (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Cruz, C. R. et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood 122, 2965–2973 (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Chung, E. Y. et al. CD19 is a major B cell receptor-independent activator of MYC-driven B-lymphomagenesis. J. Clin. Invest. 122, 2257–2266 (2012).
Haso, W. et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 121, 1165–1174 (2013).
Hegde, M. et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol. Ther. 21, 2087–2101 (2013).
Fecher, L. A., Agarwala, S. S., Hodi, F. S. & Weber, J. S. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist 18, 733–743 (2013).
Morgan, R. A. et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 36, 133–151 (2013).
Cameron, B. J. et al. Identification of a Titin-derived HLA-A1-presented peptide as a crossreactive target for engineered MAGE A3-directed T cells. Sci. Transl. Med. 5, 197ra103 (2013).
Di Stasi, A. et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med 365, 1673–1683 (2011).
Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014).
Orentas, R. J. et al. Identification of cell surface proteins as potential immunotherapy targets in 12 paediatric cancers. Front. Oncol. 2, 194 (2012).
Kloss, C. C., Condomines, M., Cartellieri, M., Bachmann, M. & Sadelain, M. Combinatorial antigen recognition with balanced signalling promotes selective tumour eradication by engineered T cells. Nat. Biotechnol. 31, 71–75 (2013).
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
All authors researched data for article, contributed to the discussion of content for the article, wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.L.M. is a co-inventor on a patent for a CD22-CAR that will soon enter clinical trials. M.S.M. and T.J.F. declare no competing interests.
Rights and permissions
About this article
Cite this article
Mackall, C., Merchant, M. & Fry, T. Immune-based therapies for childhood cancer. Nat Rev Clin Oncol 11, 693–703 (2014). https://doi.org/10.1038/nrclinonc.2014.177
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2014.177
This article is cited by
-
Pediatric medulloblastoma express immune checkpoint B7-H3
Clinical and Translational Oncology (2022)
-
Role of multidrug resistance-associated proteins in cancer therapeutics: past, present, and future perspectives
Environmental Science and Pollution Research (2021)
-
Agents in Development for Childhood Acute Lymphoblastic Leukemia
Pediatric Drugs (2018)
-
Complications of Emerging Oncology Therapies Requiring Treatment in the Pediatric Intensive Care Unit
Current Pediatrics Reports (2017)
-
Incidence, Survival, and Treatment of Localized and Metastatic Neuroblastoma in Germany 1979–2015
Pediatric Drugs (2017)