Abstract
Background
Data concerning metabolism of paracetamol in infants are scant. Previous studies have examined urinary metabolite recovery rates after a single dose of paracetamol in either neonates (<6 weeks) or children (3–9 years). There are no studies investigating infants.
Methods
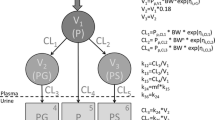
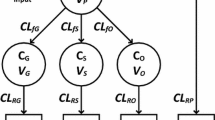
Infants (n=47) undergoing major craniofacial surgery were given paracetamol 19–45 mg/kg 6-, 8-, or 12-hourly as either elixir or suppository formulation for postoperative analgesia, after a loading dose of 33–59 mg/kg rectally during the operation. Serum was assayed for paracetamol concentration in 40 of these infants at 5, 8, 11, 14, 17 and 20 h postoperatively. Urine samples were collected every 3 h for 24 h in 15 of these infants. The clearances of paracetamol to glucuronide and sulphate metabolites as well as the urinary clearance of unmetabolised paracetamol were estimated using non-linear, mixed-effects models.
Results
Mean (±SD) age and weight of the patients were 11.8±2.5 months and 9.1±1.9 kg. Clearances of paracetamol to paracetamol-glucuronide (%CV) and to paracetamol-sulphate were 6.6 (11.5) l/h and 7.5 (11.5) l/h respectively, standardised to a 70-kg person using allometric '1/4 power' models. Glucuronide formation clearance, but not sulphate formation, was related to age and increased with age from a predicted value in a neonate of 2.73 l/h/70 kg to a mature value of 6.6 l/h/70 kg with a maturation half-life of 8.09 months. Urine clearance of paracetamol-glucuronide, paracetamol-sulphate and unchanged paracetamol (%CV) were, respectively, 2.65, 3.03 and 0.55 (28) l/h/70 kg. The urine clearance of unchanged paracetamol and metabolites was related to urine volume flow rate. Clearance attributable to pathways other than these measured in urine was not identifiable. The glucuronide/sulphate formation clearance ratio was 0.69 at 12 months of age. Sulphate metabolism contributed 50% towards paracetamol clearance.
Conclusion
Glucuronide formation clearance increases with age in the infant age range but sulphate formation does not. Renal clearance of paracetamol and its metabolites increases with urine flow rate. This and other studies show that paracetamol metabolism to glucuronide appears to be similar in infants and children, but in adults is increased in comparison with children. Oxidative pathways were undetectable in this infant study and may explain, in part, the reduced incidence of hepatotoxicity in infants.






Similar content being viewed by others
References
't Jong GW, Vulto AG, de Hoog M, Schimmel KJ, Tibboel D, van den Acker JN (2001) A survey of the use of off-label and unlicensed drugs in a Dutch children's hospital. Pediatrics 108:1089–1093
Prescott LF (1996) Paracetamol (paracetamol). A critical bibliographic review. Taylor and Francis Publishers, London
Manyike PT, Kharasch ED, Kalhorn TF, Slattery JT (2000) Contribution of CYP2E1 and CYP3A to paracetamol reactive metabolite formation. Clin Pharmacol Ther 67:275–282
Bergman K, Muller L, Teigen SW (1996) Series: current issues in mutagenesis and carcinogenesis, no. 65. The genotoxicity and carcinogenicity of paracetamol: a regulatory (re)view. Mutat Res 349:263–288
Anderson BJ, van Lingen RA, Hansen TG, Lin YC, Holford NH (2002) Acetaminophen developmental pharmacokinetics in premature neonates and infants: a pooled population analysis. Anesthesiology 96:1336–1345
van der Marel CD, van Lingen RA, Pluim MA, et al (2001) Analgesic efficacy of rectal versus oral paracetamol in children after major craniofacial surgery. Clin Pharmacol Ther 70:82–90
McGrath PA, Brigham MC (1992) The assessment of pain in children and adolescents. In: Turk DC, Melzack R (eds) Handbook of pain assessment. The Guilford Press, New York, pp 295–314
Beal SL, Sheiner LB, Boeckmann A (1999) Nonmem user's guide. Division of Pharmacology, University of California, San Francisco
Lowenthal DT, Oie S, Van Stone JC, Briggs WA, Levy G (1976) Pharmacokinetics of paracetamol elimination by anephric patients. J Pharmacol Exp Ther 196:570–578
Anderson BJ, Woollard GA, Holford NHG (2000) A model for size and age changes in the pharmacokinetics of paracetamol in neonates, infants and children. Br J Clin Pharmacol 50:125–134
Holford NHG (1996) A size standard for pharmacokinetics. Clin Pharmacokinet 30:329–332
Peters HP (1983) Physiological correlates of size. In: Beck E, Birks HJB, Conner EF (eds) The ecological implications of body size. Cambridge University Press, Cambridge, pp 48–53
Prothero JW (1980) Scaling of blood parameters in animals. Comp Biochem Physiol A67:649–657
Gabrielsson J, Weiner D (1994) Interspecies scaling. In: Gabrielsson J, Weiner D (eds) Pharmacokinetic and pharmacodynamic data analysis. Swedish Pharmaceutical Press Ltd, Stockholm, pp 153–171
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276:122–126
West GB, Brown JH, Enquist BJ (1999) The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science 284:1677–1679
van Lingen RA, Deinum JT, Quak JM, et al (1999) Pharmacokinetics and metabolism of rectally administered paracetamol in preterm neonates. Arch Dis Child Fetal Neonatal Ed 80:F59–F63
Miller RP, Roberts RJ, Fischer LT (1976) Paracetamol elimination kinetics in neonates, children and adults. Clin Pharmacol Ther 19:284–294
Alam SN, Roberts RJ, Fischer LJ (1977) Age-related differences in salicylamide and paracetamol conjugation in man. J Pediatr 90:130–135
Levy G, Khanna NN, Soda DM, Tsuzuki O, Stern L (1975) Pharmacokinetics of paracetamol in the human neonate; formation of acetaminophinglycuronide and sulphate in relation to plasma bilirubin concentration and D-glucoric acid excretion. Pediatrics 55:818–825
Anderson BJ, McKee AD, Holford NH (1997) Size, myths and the clinical pharmacokinetics of analgesia in paediatric patients. Clin Pharmacokinet 33:313–327
de Wildt SN, Kearns GL, Leeder JS, van den Anker JN (1999) Glucuronidation in humans. Pharmacogenetic and developmental aspects. Clin Pharmacokinet 36:439–452
Miners JO, Osborne NJ, Tonkin AL, Birkett DJ (1992) Perturbation of paracetamol urinary metabolic ratios by urine flow rate. Br J Clin Pharmacol 34:359–362
Barraclough MA (1972) Effect of vasopressin on the reabsorption of phenacetin and its metabolites from the tubular fluid in man. Clin Sci 43:709–713
West JR, Smith HW, Chasis H (1948) Glomerular filtration rate, effective renal blood flow, and maximal tubular excretory capacity in infancy. J Pediatr 32:10–18
Prescott LF, Wright N (1973) The effects of hepatic and renal damage on paracetamol metabolism and excretion following overdosage. A pharmacokinetic study. Br J Pharmacol 49:602–613.
Warner A (1986) Drug use in the neonate: interrelationships of pharmacokinetics, toxicity, and biochemical maturity. Clin Chem 32:721–727
Greene JW, Craft L, Ghishan F (1983) Paracetamol poisoning in infancy. Am J Dis Child 137:386–387
al Obaidy SS, Li Wan Po A, McKiernan PJ, Glasgow JF, Millership J (1995) Assay of paracetamol and its metabolites in urine, plasma and saliva of children with chronic liver disease. J Pharm Biomed Anal 13:1033–1039
Roberts I, Robinson MJ, Mughal MZ, Ratcliffe JG, Prescott LF (1984) Paracetamol metabolites in the neonate following maternal overdose. Br J Pharmacol 18:201–206
Mitchell MC, Hanew T, Meredith CG, Schenker S (1983) Effects of oral contraceptive steroids on paracetamol metabolism and elimination. Clin Pharmacol Ther 34:48–53
Sonne J, Poulsen HE, Loft S, et al (1988) Therapeutic doses of codeine have no effect on paracetamol clearance or metabolism. Eur J Clin Pharmacol 35:109–111
Mitchell MC, Schenker S, Speeg KV, Jr (1984) Selective inhibition of paracetamol oxidation and toxicity by cimetidine and other histamine H2-receptor antagonists in vivo and in vitro in the rat and in man. J Clin Invest 73:383–391
Miners JO, Penhall R, Robson RA, Birkett DJ (1988) Comparison of paracetamol metabolism in young adult and elderly males. Eur J Clin Pharmacol 35:157–160
Wynne HA, Cope LH, Herd B, Rawlins MD, James OF, Woodhouse KW (1990) The association of age and frailty with paracetamol conjugation in man. Age Ageing 19:419–424
Hoffman DA, Wallace SM, Verbeeck RK (1990) Circadian rhythm of serum sulphate levels in man and paracetamol pharmacokinetics. Eur J Clin Pharmacol 39:143–148
Baraka OZ, Truman CA, Ford JM, Roberts CJ (1990) The effect of propranolol on paracetamol metabolism in man. Br J Clin Pharmacol 29:261–264
Rumble RH, Roberts MS, Denton MJ (1991) Effects of posture and sleep on the pharmacokinetics of paracetamol (paracetamol) and its metabolites. Clin Pharmacokinet 20:167–173
Miners JO, Robson RA, Birkett DJ (1986) Paracetamol metabolism in pregnancy. Br J Clin Pharmacol 22:359–362
Miners JO, Attwood J, Birkett DJ (1984) Determinants of paracetamol metabolism: effect of inducers and inhibitors of drug metabolism on paracetamol's metabolic pathways. Clin Pharmacol Ther 35:480–486
Ismail S, Na Bangchang K, Karbwang J, Back DJ, Edwards G (1995) Paracetamol disposition in Thai patients during and after treatment of falciparum malaria. Eur J Clin Pharmacol 48:65–69
Kamali F, Thomas SH, Ferner RE (1993) Paracetamol elimination in patients with non-insulin dependent diabetes mellitus. Br J Clin Pharmacol 35:58–61
Haderslev KV, Sonne J, Poulsen HE, Loft S (1998) Paracetamol metabolism in patients with ulcerative colitis. Br J Clin Pharmacol 46:513–516
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van der Marel, C.D., Anderson, B.J., van Lingen, R.A. et al. Paracetamol and metabolite pharmacokinetics in infants. Eur J Clin Pharmacol 59, 243–251 (2003). https://doi.org/10.1007/s00228-003-0608-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-003-0608-0