Article Text
Abstract
Measurement of cerebrospinal fluid pressure through lumbar puncture (LP) manometry is an essential practical skill all paediatricians should possess competency in. The ability to perform manometry is crucial in the diagnosis of idiopathic intracranial hypertension and can provide critical information on raised (or lowered) intracranial pressure in other clinical scenarios. Practitioners should be familiar with the procedure and in particular with equipment available to them locally. In this article, we will describe an approach to LP manometry. The online supplemental material includes an instructional video as well as supporting practical information.
- Paediatrics
- Neurology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Alongside introduction of lumbar puncture (LP) in the late 19th century by Heinrich Quincke emerged a variety of techniques to measure the pressure of the cerebrospinal fluid (CSF) obtained. Apparatus resembling modern equipment with a three-way tap began to be introduced by the 1910s.1
The procedure has in essence remained unchanged for a century; a vertical hollow tube, open at its upper end, is connected via a valve to the LP needle. CSF fills the manometer until equilibrium in pressure between the CSF in the lumbar subarachnoid space and in the manometer is reached.
The height of the CSF column within the manometer gives the ‘opening pressure’, which we will call ‘CSF pressure’ for convenience. This is an estimate of intracranial pressure (ICP); discussion of how ICP relates to CSF pressure is beyond the scope of this article2; figure 1 illustrates the principles.
Lumbar puncture opening pressure is a dynamic reflection of the intra-cranial pressure, which varies with both external (eg, cardiac output and cerebral venous return) and internal factors (eg, changes in volume of the brain, blood or CSF compartments),16 assuming free flow of CSF through to the spinal subarachnoid spaces. CSF, cerebrospinal fluid.
Table 1 summarises accepted normal values, measured with the patient in the lateral decubitus position.
Normal CSF pressure range at different ages, measured in the lateral decubitus position.
When is LP manometry useful?
Knowledge of CSF pressure can add critical information in a variety of clinical scenarios (table 2), and thus manometry should be considered in any LP. Raised CSF pressure is potentially measurable before secondary signs (eg, papilloedema) present.
When is lumbar punture manometry useful?
Performing LP manometry
Preprocedure
Observe locally agreed procedural policies and preprocedure checklists (online supplemental appendix 1).
Consider following WHO Safe Surgery principles for every procedure, with a team briefing, sign in, time out, sign out and debriefing.3
Discuss informed consent with the patient and person holding parental responsibility (box 1). Ensure no contraindications to LP (box 2).
Supplemental material
Complications of LP
Complications to discuss in seeking consent for LP manometry and estimated incidence (predominantly adult data)
Back pain following (and during) procedure (~2:10,9 risk significantly increases with more than four LP attempts8).
Failure of LP and need to repeat (~1:109).
Transient nerve root irritation (~1:109).
Infection (less than 1:1008).
Bleeding, for example, spinal and subdural haematoma (less than 1:1000 to 1:200 000; risks increased with larger needle gauge, multiple needle insertions and presence of coagulopathy22).
Cerebral herniation (very low in the absence of signs of raised intracranial pressure23).
There are no specific additional risks to performing manometry.
LP, lumbar puncture.
Contraindications to LP8 23 24
Space-occupying lesion with mass effect including posterior fossa mass.
Clinical features of raised ICP (consider proceeding, with caution, if neuroimaging excludes impairment of ICP circulation and following assessment by an experienced clinician).
Pupillary dilatation (unilateral or bilateral).
Reduced pupillary light reflex.
Bradycardia.
Hypertension.
Altered breathing pattern.
Decorticate/decerebrate posturing.
Thrombocytopaenia.
Evidence is lacking to support specific LP platelet thresholds. Guidance from the British Society of Haematology suggests >40×109/L is acceptable (based largely on limited studies in stable children with haematological malignancy22), but in the context of suspected meningitis, disseminated intravascular coagulation and shock or sepsis, a higher threshold of >100×109/L is advised.
Abnormal blood coagulation.
A coagulation profile should be checked in patients with suspected increased risk of bleeding, for example, with a personal or family history of bleeding, in the context of sepsis and haematological disorders, as well as in suspected renal or liver failure.
Discuss patients with abnormal coagulation (including those with a clinically suspected bleeding disorder but normal coagulation profile) and patients taking anticoagulant medication with a haematology/clotting specialist before LP.
Infection over or around LP site.
Chiari malformation (may be safe to proceed with review of neuroimaging).
Lumbar spine structural anomalies (eg, spina bifida).
Acute or focal neurological deficit (relative contraindication — consider neuroimaging first).
Respiratory insufficiency.
Deteriorating or fluctuating GCS, or GCS score of ≤8.
At presentation with
Clinical evidence of systemic meningococcal disease.
Shock.
Seizures.
Following initial treatment and stabilisation with assessment by an experienced clinician (and if required neuroimaging), it may later be appropriate to proceed with LP.
GCS, Glasgow coma scale; ICP, intracranial pressure; LP, lumbar puncture.
Use appropriate sedation or analgesia, but be aware that moderate sedation raises ICP.4 Nitrous oxide (Entonox) increases ICP unrelated to changes in breathing.5 If used during placement of the LP needle, stop giving nitrous oxide prior to manometry.
Aim for stable end-tidal carbon dioxide (EtCO2) within a consistent normal range when general anaesthesia (GA) is used. In one study, measured CSF pressure was found to rise by 3.5–12.0 cmH2O per 1 kPa change in EtCO2; GA itself may additionally elevate CSF pressure.6
Paired blood glucose is commonly taken immediately prior to LP to avoid its false elevation from procedural stress. However, evidence to support or refute this hypothesis is scarce.
In the authors’ opinion, the most practical time to take paired blood samples is just before LP, unless the patient is likely to become too distressed by phlebotomy.
Equipment preparation
Ensure all equipment is set up in a sterile field prior to bringing the patient. Maintain aseptic technique throughout the procedure.
Although larger needle diameter increases the risk of post-LP headache, to ensure sufficiently rapid CSF flow for manometry, needles no smaller than 22 G (0.7 mm external diameter) should be used.7 8
Select a needle of appropriate length. A variety of different formulae exist to estimate the subarachnoid space depth.11 Be aware that obese patients may need longer needles than the readily available 90 mm (3.5-inch) size.
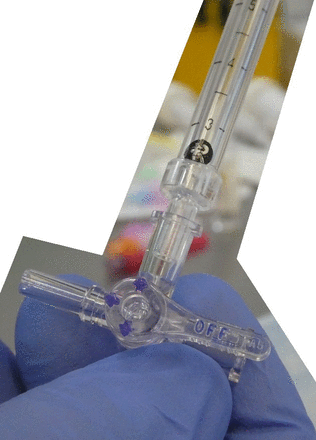
CSF manometers are usually packaged in sterile sets comprising a stackable manometer column and a three-way tap. Preassemble the manometer as shown in figure 2.
The three-way tap valve is often tight; rotate it several times to loosen it.
Attach the manometer to the three-way tap with measurement graduations facing in a direction where you will be easily able to see them.
Be certain that the manometer set and LP needle fit together before starting, even if this means opening another set. Keep it for teaching later!
Three-way tap attached to a spinal manometer. The arrows on the three-way tap indicate the direction of free flow. With the "OFF" arm pointing rightwards as shown, CSF is free to flow from the LP needle (once attached via the Luer slip connector seen here on the left) up in to the manometer column.
Positioning
Ensure your patient is comfortable laying in the lateral decubitus position with the bed horizontal.
If necessary, patients can be sat upright for LP needle placement, then carefully transferred to the lateral position. A sitting position may increase the interspinous space, raising chances of a successful LP,12 but CSF pressure in this position cannot be interpreted, and there is a risk of dislodging the needle while changing position.
Keep the neck neutral; neck flexion does not increase the interspinous distance12 and may reduce venous return from the head, increasing ICP.2
Excessive hip flexion may increase the intra-abdominal pressure and raise ICP; performing Valsalva manoeuvres (eg, coughing) has a similar effect.13 Conversely, hip extension may reduce CSF pressure by a small amount (typically less than 5 cmH2O).14
Consider beginning with the patient’s hips flexed to ease palpation of interspinous spaces, and then carefully extending the legs once the needle is sited. Beware the risk it may move out of place during this movement!
In patients who may require a subsequent LP manometry, adopt and document a replicable position to ensure the consistency of measurements.
Ensure adequate exposure of the lower back; remove or fold down clothing to ensure no obstruction to the procedure or contamination of the sterile field. Remove topical anaesthetic.
Lumbar puncture
Clean the patient’s lower back with an appropriate solution following local practices and allow time for this to dry.
Fix sterile drapes or towels to create a sterile working field.
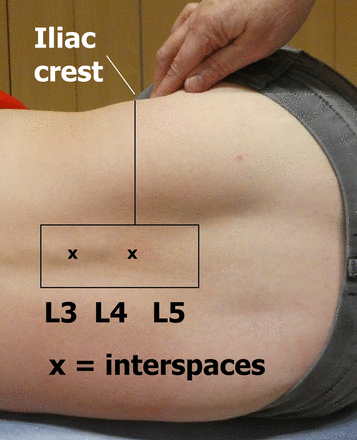
Identify the intended LP site—usually the L4–L5 or L3–L4 interspace.
The L4 spinous process approximately aligns with an imaginary line joining the superior margins of the iliac crests posteriorly (Tuffier’s line) (figure 3).
The intercristal line (Tuffier’s line) approximates to the L4 spinous process or L4–L5 interspace. The actual level may be different, depending on patient sex and body mass index.25
If using local anaesthesia (eg, lidocaine without epinephrine 1%, 0.3 mL/kg based on ideal body weight, up to a maximum of 20ml), infiltrate the subcutaneous tissue and deeper fascial tissue as appropriate, depending on the size of the patient, using a needle and syringe. The syringe should always be aspirated prior to injection to rule out accidental vascular or dural puncture. Allow time for the anaesthetic to act and warn the patient that it may cause a brief tingling sensation.
After a final check (‘time out’) that the patient, team and equipment are ready, insert the LP needle (or introducer followed by an atraumatic needle), aiming in the midline, slightly rostrally towards the umbilicus.
Avoid touching the shaft of the needle to maximise sterility; it can sometimes, however, be useful to place a finger near to the hub in order to help stabilise the needle.
Keeping the bevel of the needle perpendicular to the direction of the spinal column will encourage separation of the longitudinal fibres of the dura mater rather than dividing across them, reducing the risk of post-LP headache or CSF leak.7
Advance the LP needle until the subarachnoid space is reached.
There may be a subtle reduction in resistance to advancing the needle, but this can be hard to feel in some children.
Gradually advance the needle and keep checking whether the CSF space is entered by partially removing the stylet to check for drainage of water-like CSF or blood-tinged CSF.
It may take a few seconds for CSF to appear in the needle hub. If flow is slow, consider rotating the needle to adjust the orientation of the bevel through which CSF enters.
If no fluid appears, continue advancing until bony resistance is felt or pure blood is obtained, at which point gradual withdrawal of the needle can be attempted to see if CSF is obtained. If still unsuccessful, replace the stylet and consider a further attempt.
In the authors’ experience, the most common reasons for failure to obtain CSF are using a site too low (typically below L5) and incorrect positioning of the patient.
If CSF is seen, fully remove the stylet (keeping it safely on the sterile field) and quickly connect the three-way tap of the assembled manometer to the needle hub.
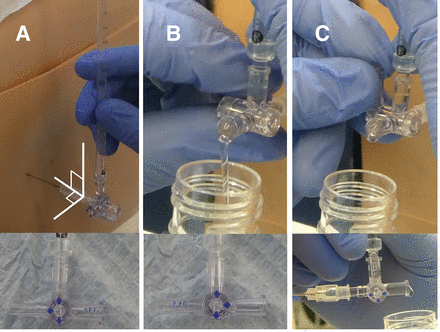
Orient the manometer column so that it is perpendicular in two planes to the ground - that is, pointing directly upwards and not angled diagonally relative to the ground (figure 4A).
(A) Opening/closing pressure measurement. Three-way valve open to patient and manometer. Note the manometer is orientated perpendicular to the ground in two planes. (B) Drainage of cerebrospinal fluid from manometer. Three-way tap valve closed to patient. (C) Sample collection bypassing manometer. Three-way tap closed to manometer.
Manometry
With the three-way tap open to the patient and the manometer (figure 4A), CSF will start filling the manometer column. The level at which the CSF meniscus stabilises should be recorded as the opening pressure to the nearest 0.5 cmH2O (figure 5).
Additional manometer columns may be necessary to record pressures greater than 40 cmH2O. An assistant with sterile gloves can stabilise these.
The meniscus will normally appear pulsatile; this reflects changes in CSF pressure with breathing and the cardiac cycle.
Recording of opening/closing pressure—here the meniscus would be recorded at 22.5 cm H2O.
To measure the CSF pressure most accurately, the opening pressure should be observed and readings repeated over a period of time. Short-term monitoring over at least 20 min and recording the mean average pressure may provide more reliable CSF pressure measurement.15
This process may be time-consuming and not feasible for all patients—aim to record for as long as is practical and interpret the results in the wider clinical context. We discuss monitoring of dynamic CSF pressure further in online supplemental appendix 2.
Collect CSF samples—first empty the manometer (figure 4B), then either remove the manometer assembly and collect samples from the LP needle as usual, or leave it attached and close the three-way tap to the manometer (figure 4C).
In patients who are likely to move while collecting CSF, removing the manometer at this time may reduce the risk of accidentally dislodging the LP needle. Take care not to move the needle when re-attaching the manometer.
CSF neurotransmitters must be collected before manometry.
Following sample collection, measure the closing pressure using the same method as for the opening pressure (figure 4A)
Take care to ensure the actual meniscus is measured rather than from air bubbles or any fluid that remained in the manometer.
If the closing pressure is higher than desired, then remove further CSF in 2.5–10.0 mL volumes incrementally (box 3) using the same method as for CSF test samples. Repeat closing pressure measurement and repeat until the target closing pressure is achieved (box 4).
How much CSF can be safely collected?
In adults, with a typical cranial CSF volume of at least 150 mL,26 it has been suggested that collection of up to 30 mL is safe8; CSF is synthesised at a rate around 0.35 mL/min,16 and so this volume will be replaced within a few hours at most.
By contrast, the total cranial CSF volume of neonates is around 40 mL at term,27 rapidly increasing in the first 1–2 years of life.26 There are no studies examining the maximum volume of CSF which can safely be taken from a neonate or infant, outside the context of hydrocephalus.
Beyond the age of around 2 years, MRI studies suggest cranial CSF volume is similar to adults,26 and thus collection of 30 mL is unlikely to be problematic.
CSF, cerebrospinal fluid.
Target closing pressure in IIH
IIH is a condition with raised ICP without a structural cause (eg, tumour or hydrocephalus) and normal CSF composition and can be defined by Friedman’s diagnostic criteria. It is also known as pseudotumour cerebri syndrome and was previously called benign intracranial hypertension. Patients typically present with headaches and may have visual symptoms and signs on examination, typically including papilloedema, with an otherwise normal neurological examination.19
When performing an LP for diagnosis of IIH, if CSF pressure is found to be raised, aim for a closing pressure within the normal range (eg, between 20 cmH2O and 25 cmH2O in most children above the age of 1 year).19
Some clinicians suggest not reducing pressure to less than 30 cmH2O if CSF pressure is exceptionally high (ie, greater than 60 cmH2O) to avoid risks of post-LP headaches, but this is not evidence-based.7 There is no agreed position on the management of such patients who are symptomatic for IIH but are found to have normal opening pressure.
CSF, cerebrospinal fluid; ICP, intracranial pressure; IIH, idiopathic intracranial hypertension; LP, lumbar puncture.
Postprocedure management
Detach the manometer assembly; replace the stylet and remove the LP needle, with application of a sterile dressing as per local policies.
Fully document patient position and other factors which may have influenced CSF pressure (eg, sedation, blood pressure, pCO2 and psychoactive medications). Document the opening and closing pressures along with the volume of CSF removed.
Evidence does not support that bed rest, laying flat, consumption of fluids or caffeine will reduce risks of post-LP complications.7
Arrange appropriate management and follow-up, depending on the LP findings and clinical scenario.
Conclusion
LP manometry in a cooperative patient is a straightforward addition to a standard LP, which can provide invaluable insight to a patient’s underlying diagnosis.
To carry out the procedure well takes practice. We would recommend that practitioners attempt manometry observing this, or similar guidance, in their ‘routine’ LPs, so that when pressure measurement is critical for diagnosis (such as in idiopathic intracranial hypertension), they can confidently perform the technique.
Clinical bottom line
Lumbar puncture (LP) manometry is a simple addition to standard LP, which does not introduce additional risks.
LP manometry provides essential information for the diagnosis of idiopathic intracranial hypertension and spontaneous intracranial hypotension.
It may also provide useful information in the management of a range of other neurological conditions.
Practitioners should be actively aware of factors influencing LP opening pressure in order to record accurate measurements and take efforts to minimise their impact.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
The authors wish to thank the patients and their family who gave consent for filming of their lumbar puncture (LP) for educational purposes. We also wish to thank in particular Dr Yetunde Arulogun and Dr Pooja Harijan, who assisted in the production of the LP manometry video available in the online supplemental materials. Additional thanks to the Cambridge Clinical Skills Laboratory for use of their LP mannequin, and to Angela Curtis and Sharon Whyte for their input.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors All authors conceived the article. JAAH and JF wrote and edited the draft, which was reviewed by all authors. DK is the guarantor.
Funding JAAH is funded by an Action Medical Research and British Paediatric Neurology Association Research Training Fellowship.Many thanks to IIH UK for funding the Article Processing Charge, enabling open access publication.
Disclaimer The funders have had no involvement in the creation of this article.
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.