Article Text
Statistics from Altmetric.com
Introduction
‘Calcitonin’ was first identified during studies of parathyroid function in dogs1 – a new parathyroid hormone that caused a transient hypocalcaemia. Subsequently demonstrated in the human serum of patients with medullary thyroid carcinoma, its heterogeneity hinted at the existence of multiple forms of this new hormone and of a precursor prohormone.2 The identification of this procalcitonin (PCT) in the hypocalcaemia of Staphylococcal toxic shock syndrome3 first drew the association of PCT with sepsis and inflammatory states, which was confirmed by subsequent studies. The first prospective study of PCT in children with sepsis revealed a rapid and dramatic increase in the levels of PCT, which normalised with appropriate antibiotic therapy.4 Elevated PCT has been demonstrated following the injection of endotoxin into healthy volunteers, that is detectable at 4 h with a peak at 6 h postadministration.5
Demonstration of its rapid increase in response to inflammatory stimuli and favourable comparisons of its performance as an indicator of serious infection with established markers such as C-reactive protein (CRP) have encouraged the ongoing investigation of PCT as a reliable and discriminatory diagnostic and prognostic tool in childhood and adult infection. Furthermore, the study of PCT has begun to explain its role as a mediator of sepsis. Antibodies that bind PCT have markedly reduced mortality from sepsis in animal models suggesting that immunoneutralisation may be a promising adjunctive therapy.6 This paper seeks to elucidate the current understanding of PCT as a marker of childhood infection and to draw attention to areas of ongoing research.
Physiological background
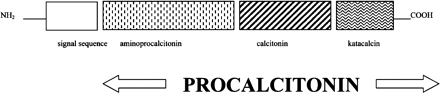
PCT is a 116 amino acid protein derived most commonly from neuroendocrine cells including the C-cell of the thyroid with which it was first associated. While calcitonin is thought to have a role in calcium metabolism and bone resorption through its action on the osteoclast,7 the action of PCT remains unclear. This prohormone of calcitonin consists of a 57 amino acid residue at the amino terminus (amino-proCT), the immature 33 amino acid calcitonin peptide and a 21 amino acid carboxy-terminal (CCP-I or katacalcin) (figure 1). In normal conditions, the calcitonin gene CALC-I, located on chromosome 11, is selectively expressed in neuroendocrine tissues and transcribes the prohormone PCT. In these circumstances, calcitonin is then produced by the action of proteolytic enzymes on PCT. PCT and its constituent proteins are present in low concentrations in normal subjects and become markedly elevated in a number of conditions (table 1). Under such conditions, including sepsis, CALC-I is expressed systemically and calcitonin mRNA detected in a wide variety of tissues.8 The resultant increase in PCT occurs in the absence of the enzymatic pathways that control calcitonin and its precursors in the neuroendocrine tissue and is not mirrored by a corresponding increase in the calcitonin level.
Schematic diagram of the procalcitonin molecule. Reproduced with permission from Carrol ED et al34
Conditions that elevate PCT
Technological background
A number of commercial assays for PCT exist. Each uses a sandwich immunoassay technique in which two distinct monoclonal antibodies attach specifically to different regions of the PCT molecule. Two quantitative and one semiquantitative assay have been widely studied, each with its own functional sensitivity and test characteristics. In the established immunoluminometric assay (ILMA), the first antibody specific to the CCP-I region is immobilised in a ‘coated tube’, while the second antibody attaches to the carboxy-terminal of the calcitonin molecule. This second antibody is labelled with a luminescent tracer and in the presence of PCT, the tracer then becomes bound to the coated tube in a ‘sandwich complex’ (figure 2). The luminescence signal generated is directly proportional to the concentration of PCT in the sample and by measuring the signal with a luminometer and comparing it to a standard curve, PCT can be quantified. The functional assay sensitivity (FAS) of the ILMA technique is approximately 0.3 ng/ml. Newer, more sensitive assays such as Kryptor (Thermo Fisher, Hennigsdorf, Germany) or VIDAS B.R.A.H.M.S PCT (Biomerieux, Marcy l’Etoile, France) can achieve a rapid quantification of PCT with FAS to 0.06 ng/ml. A rapid semiquantitative point of care test exists in which the sandwich complex forms to produce a colour change in the test band. This colour change relates to broad ranges of PCT concentration – PCT < 0.5 ng/ml, 2.0 > PCT > 0.5 and 10.0 > PCT > 2.0 and PCT > 10.0 ng/ml (PCT-Q, Thermo Fisher).
An illustration of the sandwich technique as an example of an immunoassay.
When interpreting studies of PCT, consideration needs to be given to the assay used. Most studies of the performance of PCT as a marker of sepsis have utilised the ILMA with its functional sensitivity of 0.3 ng/ml. This level exceeds normal, as determined by a highly sensitive research tool, by a factor of 10-fold and thus fails to identify smaller elevations in PCT.9 A recent comparison of the semiquantitative PCT-Q and the sensitive Kryptor assay revealed only a moderate agreement between the two.10 While previous studies have revealed good concordance when there are few technicians recording the results of the PCT-Q test, the authors suggest that their findings – in which many different laboratory technicians read the results of the semiquantitative test in the course of a busy clinical environment – highlight the subjectivity of PCT-Q. Inconsistent reports of the usefulness of PCT may in part occur as a result and future studies should aim to use the more sensitive quantitative techniques.
Indications and limitations
Can PCT identify serious bacterial infection in the clinically unwell neonate?
Neonatal sepsis represents a substantial burden of neonatal morbidity and mortality. The use of PCT to identify sepsis in this context is complicated by a physiological rise in both term and preterm, healthy infants, which peaks at approximately 24 h and returns to normal by day 3.11 12 Furthermore, common pathologies, particularly of the preterm neonate – such as respiratory distress syndrome – may induce a rise in PCT. Moreover, the vulnerability of the neonatal population to infection and the importance of prompt antimicrobial therapy necessitate a test of high sensitivity with the ability to confidently ‘rule out’ sepsis. Despite this, the earliest studies of PCT in neonates revealed it to be a promising indicator of bacterial infection. A single CRP measurement failed to identify 4 of 18 septic neonates (>32 weeks) in a Paris neonatal intensive care unit (NICU), whereas all were identified by PCT.13 Using a control group of 83 well, term neonates, Chiesa et al12 defined PCT reference ranges for each hour of age, then reported a sensitivity of 85.7% (24/28) for a value above the age appropriate upper limit to detect early onset sepsis (EOS). In contrast, only 13 of 28 neonates had an initially elevated CRP, though in the following 48 h CRP increased above 10 mg/dl in all. Subsequent studies re-affirmed the superiority of PCT over CRP in identifying EOS14 and late onset sepsis (LOS)15 though the performance of the test varied considerably. While Jacquot et al15 identified all 30 neonates with a confirmed LOS using a PCT threshold of 0.6 ng/ml, a multi-centre study from Spain applying a similar threshold achieved a sensitivity of 81.4% and negative predictive value of 72.5% and concluded that PCT remains an insufficient tool in isolation.16 Interestingly, the latter of the two studies used an ILMA while the former used the highly sensitive Kryptor assay.
PCT performs better than CRP in the identification of neonatal sepsis. A PCT assay with a high sensitivity would offer the greatest prospect of success in supporting clinicians to rule out sepsis in this group.
In children with fever without a source presenting to the emergency department, does PCT identify those with serious bacterial infection?
Fever is commonly a cause of presentation to the emergency department (ED) and though most children with a serious bacterial infection (SBI) will be identified by a thorough history and clinical examination, a substantial number of children who present with fever and no apparent source will have SBI. Clinical scores have been reported to perform variably when discriminating between serious and self-limiting infection and attention has been focused on laboratory markers to support clinical decision making.
A summary of the prospective studies of PCT as a marker of SBI in the ED is shown in table 2.17,–,22 While the incidence of SBI in febrile children attending the ED is estimated to be 5%, studies of laboratory tests including PCT have focused on those children who, following a thorough clinical assessment, are considered to need further investigation. The inclusion criteria for each of the studies varied, but all selected a group who were considered to be at risk of SBI, and this is evident in the range of SBI incidence reported (18–59%). Laboratory testing aims to address diagnostic uncertainty by confidently ruling out SBI or conversely by ‘ruling in’ SBI and influencing a decision to admit to hospital or commence antibiotics. Correspondingly, the table summarises the post-test probabilities following ‘positive’ or ‘negative’ tests at the thresholds defined by each study.
Studies of children presenting to the ED with fever without source
PCT performs moderately but consistently better than CRP in studies of children presenting to the ED with fever without a source (FWS).17,–,22 Furthermore, PCT consistently performs better in children presenting with a short history (<12 h) of fever, when CRP is unreliable.20,21 None of the studies specifically addressed the different priorities of ruling in or ruling out SBI. Applying a single fixed cut-off for the diagnosis of SBI may limit the interpretation of the test result. For example, by applying a cut-off of 0.12 ng/ml to the population of young infants studied by Maniaci et al,22 a negative result reduced the likelihood of SBI from 18% to 4%. This may be considered an acceptable risk to the clinician considering discharging a child from hospital. A positive test at this level, however, only increased the likelihood of SBI marginally to 22% adding little to the decision to treat. In the same way, in the study by Andreola et al,21 while a positive test at a cut-off of 0.8 ng/ml increased the likelihood of SBI significantly (from 23% to 59%), a negative test at this threshold was still associated with a risk of SBI of almost 10%. In order to optimise the utility of the test, different cut-off values should be applied reflecting the clinical priority.
Combining biomarkers may enhance the ability of clinicians to identify serious infection. Recently, a simple ‘lab-score’ was described combining PCT, CRP and urinalysis (a positive leucocyte esterase or nitrite result). In combination, the sensitivity and specificity for SBI of a lab-score ≥3 was superior to that of PCT or CRP alone.23 This was subsequently validated in an independent dataset.24
Identifying SBI in children presenting with FWS is challenging. No single clinical or laboratory test yet identifies all SBI, nor is able to entirely exclude it. PCT in association with a thorough clinical assessment performs better than CRP and is likely to be enhanced in combination with validated clinical scores.
Differentiating viral from bacterial infection: can PCT differentiate between a viral and bacterial aetiology in children presenting clinically with lower respiratory tract infection?
An area of interest with regard to the differentiation of a viral or bacterial aetiology is community acquired pneumonia (CAP). Moulin et al25 described a significant difference in PCT among 72 children diagnosed with viral or bacterial pneumonia (a further 16 were not analysed owing to a failure to make an aetiological diagnosis) and revealed an area under the curve of 0.93. Toikka et al,26 however, showed a significant overlap in the value of PCT between two groups of children with CAP of viral or bacterial origin and concluded that there was only limited value of the test in differentiating the two. Numerous studies have reported conflicting results in relation to the ability of PCT to differentiate between viral and bacterial causes of CAP. One reason for this is the lack of a gold standard test against which to compare.
Does PCT identify pyelonephritis in children presenting with febrile urinary tract infection?
Upper urinary tract involvement in urinary tract infection (UTI) may lead to renal scarring and longstanding sequelae. Differentiating lower UTI from pyelonephritis is challenging clinically and is necessary to guide appropriate duration (and possibly administration) of therapy. Despite its limitations, dimercaptosuccinic acid (DMSA) scintigraphy is considered the gold standard for the diagnosis of acute pyelonephritis and for the subsequent identification of renal scarring but is expensive and exposes children to ionising radiation. One meta-analysis and one narrative review recently evaluated the use of PCT to predict those children with UTI with an involvement of the upper tract.27 28 Each concluded that PCT predicted the presence of acute lesions on DMSA reasonably well. Of the 10 studies included in the meta-analysis (n=627), eight concluded that PCT at a threshold of 0.5–0.6 ng/ml was discriminatory, with a pooled diagnostic OR of 26.73 (95% CI 10.29 to 69.39). There was marked heterogeneity between the 10 studies, however, limiting the conclusions of the analysis. Where PCT was compared with other markers such as CRP, it performed better. The value of predicting acute changes on DMSA is questionable, however, this is a test that few UK clinicians would apply acutely. Only one study also considered the more significant clinical outcome of persistent lesions on later imaging, demonstrating that those acute lesions associated with a low initial PCT were unlikely to persist. The use of PCT in febrile children with UTI may then help to stratify management and to identify those children likely to need subsequent investigation.
Does the use of PCT as a diagnostic test improve rational antibiotic prescribing in children?
Antibiotic stewardship describes the processes involved in optimising the use of antimicrobials. Exposure to antimicrobials drives the selection of resistant organisms and in the context of accelerating antimicrobial resistance globally, there has been a progressive decline in investment into new antibiotics. Intervention studies, looking at antimicrobial stewardship programmes in children, have revealed that improved diagnostic testing had the greatest impact on antimicrobial prescribing.29 In adults, the use of PCT-guided therapy reduced antibiotic prescribing in patients hospitalised with severe CAP.30 Recently, a meta-analysis of PCT-guided therapy in the intensive care unit (ICU) (including one study of PCT-guided prescribing in NICU) demonstrated a significant reduction of approximately 2 days in the duration of antibiotics prescribed for an initial infective episode and of 4 days total exposure to antibiotics. There was no difference in outcomes such as 28-day mortality or length of stay.31 In a study of EOS in NICU, neonates (>34 weeks gestation) commenced on antibiotics with a suspicion of sepsis were randomised to standard therapy or PCT-guided therapy. In the PCT-guided group, antibiotics were discontinued following two consecutive levels below (arbitrarily defined) age-specific cut-off values. The duration of antibiotics in the standard group was determined by the likelihood of serious infection. The proportion of neonates who received >72 h antibiotics was significantly reduced in the PCT-guided group and the mean duration of antibiotics was shortened by 22 h (p=0.012).32 Only one other study has so far tested the hypothesis that PCT-guided therapy in children might reduce antibiotic prescribing. In this, children presenting to the ED with fever without source were randomised to PCT-guided therapy or PCT-blinded therapy. Comparing two groups of 192 children, there was no difference in the number of prescribed antibiotics by clinicians blinded to the PCT result compared with those whose treatment was determined in the light of a PCT result. However, the test used was the semiquantitative PCT-Q.33
Areas of ongoing interest
There is substantial evidence to support the utility of PCT in the diagnosis of SBI in children. The National Institute for Health and Clinical Excellence (NICE) provides guidance to National Health Service clinicians in the UK. In its clinical guideline ‘feverish illness in children’, NICE failed to recommend its use on a limited economic analysis based on the study of semiquantitative PCT by Galetto-Lacour et al.18 A comprehensive analysis applying data on the incidence of SBI in febrile children (the analysis estimated an incidence of SBI of 5% though the incidence of SBI in the group of children in which clinicians use laboratory tests is substantially higher) and updated costs (CRP estimated to cost £1.50 per test, PCT estimated to be £9) and utilising data from subsequent studies of a highly sensitive PCT assay would be helpful.
Adult studies have demonstrated the potential for PCT to guide rational antibiotic prescribing in CAP and in the ICU.30 31 Further study of PCT-guided algorithms in children and neonates will clarify the role of PCT in this context. Once again, highly sensitive assays appear more likely to offer an effective tool, while semiquantitative assays seem limited. The development of a highly sensitive point of care test could have a role in limiting antibiotic exposure in apparently well children in primary care and this hypothesis should be tested in an appropriately designed randomised controlled trial.
Outside the scope of this review of PCT as a diagnostic tool, but an area of significant interest in relation to the potential management of sepsis in children, is the question of the role of PCT as a mediator of sepsis. PCT injected into the peritoneum of hamsters with Escherichia coli induced sepsis increased mortality to almost 100%, while administration of a goat antiserum to PCT markedly improved outcome. This finding has been replicated in pigs.6 The implication that the immunoneutralisation of PCT or its component peptides may have a therapeutic role in sepsis warrants further exploration.
Summary (clinical bottom line)
▶ PCT is a sensitive and specific marker of SBI in children. It is better able to discriminate SBI than CRP and is particularly useful in the early stages of infection. Like other markers of infection, it is unable to rule in or rule out sepsis in all febrile children.
▶ Applying age-specific normograms of PCT in the immediate neonatal period, it is possible to identify neonates at risk of EOS. In this vulnerable group, it would appear that the role of PCT would be to support decisions to limit the duration of antibiotics commenced on suspicion of sepsis.
▶ PCT has the ability to discriminate between viral and bacterial infections in children, though there is significant overlap in the value of PCT between the two groups. In the context of CAP, there is limited evidence for the role of PCT, though future studies would benefit from the use of a validated ‘gold standard’ for the diagnosis of bacterial pneumonia.
▶ PCT identifies pyelonephritis in children presenting with a febrile UTI and predicts the subsequent development of renal scarring. It may have a role in determining those children with UTI who need subsequent evaluation with DMSA.
▶ Antibiotic stewardship is a public health priority and is improved in children by access to high-quality diagnostic tests. PCT-guided algorithms may reduce prescribing in NICU and further studies should test its utility in other paediatric clinical contexts.
References
Footnotes
-
Funding ADI is funded by the NIHR Research for Innovation, Speculation and Creativity Programme.
-
Competing interests None.
-
Provenance and peer review Commissioned; internally peer reviewed.