Article Text
Abstract
Paroxysmal cold haemoglobinuria (PCH) accounts for around a third of cases of autoimmune haemolytic anaemia in children. PCH is caused by an autoantibody that fixes complement to red cells at low temperatures and dissociates at warmer temperatures (a biphasic haemolysin), triggering complement-mediated intravascular haemolysis. Named the Donath-Landsteiner (D-L) antibody after its discoverers, it is usually formed in response to infection and demonstrates specificity for the ubiquitous red cell P-antigen. A D-L test can be used to detect the presence of the D-L autoantibody in the patients’ serum. Here we discuss the use of the D-L test in identifying PCH in a 2-year-old boy who presented with haemolytic anaemia. A summary of the key information can be found in the infographic.
- biochemistry
- jaundice
- physiology
- pathology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Case history
History: A 2-year-old previously healthy boy presented with a 24-hour history of increased drowsiness, jaundice and generalised abdominal pain on a background of a recent coryzal illness within the last 2 weeks. Parents reported his urine was dark brown/red like ‘Coca-Cola’. There was no history of recent travel, ingested toxins or diarrhoea. The patient had an up-to-date vaccination history, no medical history or family history of haemoglobinopathies.
Physical examination: On examination, he was lethargic, cold peripherally (temperature 37.2°C) with a thready pulse, had pale conjunctiva and tachycardia (HR 166 beats/min). Respiratory rate was 38 breaths/min. A systolic flow murmur was present. There was no evidence of a rash, petechiae or bruising, no palpable hepatosplenomegaly and no neurological deficits.
Investigations: Preliminary investigations included a septic screen (blood culture, urine dipstick and microscopy and a blood gas), full blood count (FBC) and film, U&E and LFTs. The initial FBC (figure 1) and film suggested a haemolytic anaemia within an hour. Further bloods included coagulation and a haemolytic screen (haptoglobin, split bilirubin (conjugated and unconjugated), reticulocyte count, lactate dehydrogenase (LDH) and a direct antiglobulin test (DAT) to look for an immune cause).1 In an attempt to identify an infective cause, testing for syphilis, cytomegalovirus, parvovirus, respiratory syncytial virus, coxsackie virus and mycoplasma pneumoniae was performed.
Interpretation: Acute intravascular haemolysis: severe anaemia, hyperbilirubinaemia, raised LDH, low haptoglobin and haemoglobinuria. Renal function, coagulation screen, platelets and white blood cells were within normal values. A DAT-positive for complement (C3) and negative for IgG showed that the red blood cells were coated with C3 and suggestive of paroxysmal cold haemoglobinuria (PCH) or a cold autoimmune haemolytic anaemia such as cold agglutinin disease1 (figure 1). The blood film obtained on day 1 showed spherocytes and red cell agglutination also in keeping with a haemolytic picture (figure 2). His blood cultures, virology and bacterial screen were negative and did not identify a cause.
Column 1 shows the initial investigations for AIHA in a patient presenting with a haemolytic picture. Column 2 shows results from the initial investigations of the patient. Column 3 shows reference ranges. AIHA, autoimmune haemolytic anaemia; CMV, cytomegalovirus; DAT, direct antiglobulin test; FBC,full blood count; LDH, lactate dehydrogenase; LFT, liver function test; RBC, red blood cell; RSV, respiratory syncytial virus; U+E, urea and electrolyte.
Red cell agglutination (A) and spherocytes (B) in the peripheral blood film of the 2-year-old patient on day 1 of his presentation with AIHA. Spherocytes are RBCs that are smaller and denser than normal RBCs due to their sphere shape rather than the RBCs’ characteristic biconcave shape. Spherocytes in a blood film are most often associated with an immune-mediated haemolytic anaemia.23 AIHA, autoimmune haemolytic anaemia; RBC, red blood cell.
Case history
Further testing: A positive indirect Donath-Landsteiner (D-L) test confirmed the diagnosis of PCH and the presence of D-L antibodies (figure 3).
The patient’s positive D-L test. Tube 1: the patient’s serum+donor fresh serum+reagent P-antigen-positive group O RBC incubated at 37°C. Tube 2: duplicate of tube 1 but double incubation at 0°C and 37°C. Tube 3: the patient’s serum+donor fresh serum+reagent P-antigen-positive group O RBC treated with enzyme 1% papain at 37°C. Tube 4: duplicate of tube 3 but double incubation at 0°C followed by 37°C. Tubes 2 and 4 both show haemolysis and thus a positive D-L test ( √ ) confirming the presence of PCH. Methodology: patient’s fresh serum (2 mL minimum, with a 0.5 mL EDTA sample for grouping) was collected and maintained at 37°C (immerse blood sample tube immediately in thermoflask filled with 37°C water for transportation). Reagent P-antigen-positive group O RBC and fresh donor serum (source of complement since patients with PCH may have low complement due to prior consumption) were added. The sample was separated and subjected to heating under three conditions: (1) 4°C for 90 min, (2) 37°C for 90 min and (3) 4°C for 30 min followed by 37°C for 60 min. A positive D-L test and diagnosis of PCH are demonstrated when haemolysis only occurs after condition 3).16 To increase sensitivity, enzymes such as 1% papain can be used to expose more of the RBC surface P-antigens to increase possibility of binding with D-L autoantibodies.1 D-L, Donath-Landsteiner; PCH, paroxysmal cold haemoglobinuria; RBC, red blood cell.
Background
Haemolytic anaemias occur in two major types according to aetiology: intrinsic and extrinsic.2 Intrinsic haemolytic anaemias are usually inherited and caused by a defect in the red blood cell (RBC) itself leading to premature RBC haemolysis. Extrinsic haemolytic anaemias are usually acquired and involve the premature destruction of the RBC by external factors such as antibodies, infections or trauma. Autoimmune haemolytic anaemia (AIHA) is an extrinsic haemolytic anaemia characterised by the production of autoantibodies that bind to the surface of RBCs leading to premature haemolysis.
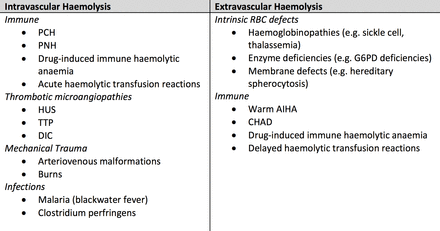
Haemolysis of RBCs can occur via two mechanisms: intravascular, the destruction of RBCs in the circulation; and extravascular, the destruction of RBCs by macrophages of the spleen, liver or bone.2 Laboratory results and clinical manifestations can help differentiate between the mechanism of haemolysis. Haemolysis in AIHA can either be intravascular or extravascular, although paroxysmal cold haemoglobinuria (PCH) is almost exclusively intravascular3–5 (figure 4).
Some examples of the causes of intravascular and extravascular haemolyses to be considered in a child presenting with a haemolytic picture.5 AIHA, autoimmune haemolytic anaemia; CHAD, cold agglutinin disease; DIC, disseminated intravascular coagulation; G6PD, glucose 6 phosphate dehydrogenase; HUS, haemolytic uraemic syndrome; PCH, paroxysmal cold haemoglobinuria; PNH, paroxysmal nocturnal haemoglobinuria, RBC, red blood cell; TTP, thrombotic thrombocytopenic purpura.
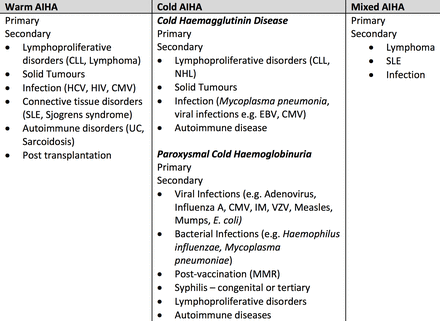
Cases of AIHAs are generally classified according to the optimal temperature the autoantibodies bind to the patients’ RBCs: warm AIHA (37°C), cold AIHA (<37°C) and mixed AIHA1 3 6 (figure 5). There are two distinct AIHAs associated with cold-reactive autoantibodies: cold haemagglutinin disease (CHAD) is more common in adults and PCH is significantly more common in children.3 The aetiology of AIHAs can be categorised as primary (idiopathic, approximately 50%) and secondary to underlying disorders such as malignancy, infections or autoimmune diseases.6 7
Classification of AIHAs and associated aetiologies.2 6 AIHA, autoimmune haemolytic anaemia; CLL, chronic lymphocytic leukaemia; CMV, cytomegalovirus; EBV, Ebstein-Barr virus; HCV, hepatitis C virus; IM, infectious mononucleosis; MMR, measles, mumps and rubella; NHL, non-Hodgkin’s lymphoma; SLE, systemic lupus erythematosus; UC, ulcerative colitis; VZV, varicella zoster virus.
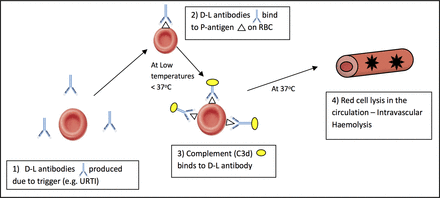
Although AIHAs are rare in children in the UK, they result in lengthy admissions and carry potential morbidity if not correctly identified. The incidence of AIHA in children has been estimated at greater than three per million/year and possibly as high as 1 per 100 000/year.8 9 Studies estimate that 20%–50% of paediatric AIHA is caused by PCH.10 11 PCH was first described in 1872, when most cases were associated with congenital syphilis.12 PCH in children now more commonly occurs as an acute transient haemolysis secondary to infections (>70% post respiratory infection), vaccinations, haematological malignancies and autoimmune disorders (figure 6).1 4 12–14 Often the haemolysis in PCH is severe because it is intravascular; however, spontaneous resolution over several weeks, once the infection has subsided, is common.15
Suggested haemolysis in paroxysmal cold haemoglobinuria. D-L, Donath-Landsteiner; RBC, red blood cell; URTI, upper respiratory tract infection.
The autoantibody associated with PCH is the D-L antibody after its discoverers in 1904. An indirect D-L test is the only specific diagnostic tool available for PCH.16 The D-L antibody is classically a polyclonal biphasic IgG autoantibody with specificity for the RBC P-antigen4; other non-IgG D-L antibodies, IgA and IgM, have been reported.17 18 At low temperatures, <37°C, the D-L antibody fixes complement to RBCs and on heating to 37°C triggers complement-mediated lysis (figure 6), as the D-L bound RBCs travel from the peripheries to the core.
Difficulties associated with the D-L test have led to controversies regarding the actual incidence of PCH. The D-L test is technical, often carried out incorrectly and may be missed in the diagnostic tests for AIHA.19
Clinically relevant questions
How might a child with AIHA present clinically?
Children with AIHA commonly present with symptoms and signs of acute haemolysis: anaemia (weakness, dizziness, pallor and dyspnoea) and haemolysis (jaundice).1 Non-specific symptoms may include fever, malaise, confusion, abdominal pain and back or leg pain.11 Depending on the severity of the anaemia, tachycardia and systolic flow murmurs may occur. Other physical examination findings may suggest associated conditions and/or the underlying cause, for example, lymphadenopathy and/or hepatosplenomegaly suggestive of an underlying malignancy or bruising and petechiae associated with coagulation abnormalities such as in thrombotic microangiopathy (TMA).
Examination findings may vary with the type of haemolysis involved. Hepatosplenomegaly is primarily associated with extravascular haemolysis and haemoglobinuria occurs with intravascular haemolysis, such as in PCH, often presenting as a dark/red coca-cola coloured urine.10 20
What initial laboratory tests may be useful in aiding a diagnosis of haemolytic anaemia and what might you expect from these investigations?
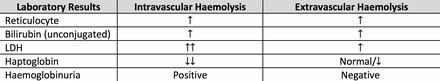
A haemolytic screen should be carried out and its result interpreted collectively: full blood count (FBC) and film, lactate dehydrogenase (LDH), split bilirubin (unconjugated and conjugated), reticulocytes, haptoglobin and urinalysis (dipstick and microscopy).21 Typical findings and limitations in interpretation are noted in figure 7. Laboratory results in extravascular haemolysis are generally less severe, as haemolysis occurs in the reticuloendothelial system, with fewer degradation products released into the circulation.21
Comparison between the typical laboratory findings in an extravascular versus an intravascular haemolysis.22 Note: Haptoglobin is not produced efficiently in children under 2 years or those with liver disease (levels are artificially low) and rises with acute inflammation, so it may be misleadingly normal in infection/inflammation despite haemolysis. LDH is non-specifically elevated in tissue damage and may remain low in extravascular haemolysis as red cells are mopped up by the reticuloendothelial system. Reticulocytes may be depressed initially because infection is often myelosuppressive and then rebound. ↑↑, significantly increased; ↑, increased; ↓, decreased; ↓↓, significantly decreased; LDH, lactate dehydrogenase.
Haemolysis causes anaemia if the bone marrow RBC production cannot compensate for increased RBC destruction. Haemoglobinuria should be suspected if the urine dipstick is positive for blood but urine microscopy shows minimal red cells. Haptoglobin, the most sensitive laboratory indicator of haemolysis and last marker to normalise, is significantly decreased due to free haemoglobin (Hb) exceeding the Hb-binding capacity of the circulating haptoglobins.5 A reticulocytosis is usually seen, although with AIHA reticulocytopenia can commonly occur in the acute phase due to myelosuppression secondary to a recent infection or inadequate compensatory reticulocytosis.4 10 The patient’s blood results are shown in figure 1.
Can peripheral blood films help distinguish between the type of haemolysis (ie, intravascular or extravascular) and are there any characteristics associated with AIHA?
Non-specific characteristics of blood films associated with AIHA include red cell agglutination and anisopoikilocytosis (varying size and shape of RBCs) such as schistocytes, spherocytes and polychromasia, although these can also be associated with other causes of haemolytic anaemia, for example, mycoplasma infections and lymphoproliferative disorders.22 Spherocytes tend to be a characteristic of extravascular haemolysis while intravascular haemolysis is associated with schistocytes.23 The blood film from the 2-year-old boy, showing spherocytes and red cell agglutination, did not show any specific changes suggestive of an intravascular haemolysis (figure 2).
How might you differentiate between non-immune and immune causes of haemolysis and when might you consider a D-L test for suspected PCH?
The cause of haemolysis may be obvious and the clinician can proceed directly to specific diagnostic testing, for example, haemolysis with blood film characteristics of sickle cell or thalassemia.20 Other blood tests platelets, lymphocytes and granulocytes, renal function and coagulation screens can provide diagnostic insight and significant derangements with haemolysis may suggest more serious underlying causes, for example, TMA or clonal haematopoietic disorders.
If the cause of haemolysis is less clear, a direct antiglobulin test (DAT) can determine whether the haemolysis is immune or non-immune.20 24 In the DAT screen, human antibodies against C3 and IgG are incubated with the patients’ RBCs. If the surface of the RBCs is coated with IgG or C3, agglutination occurs and a DAT-positive test suggests an immune aetiology.24 DAT-positive tests can occur in other non-haemolytic diseases e.g. SLE, malignancy or renal disorders.1 Figure 8 reveals the common DAT findings in AIHA.1 A DAT-negative test does not exclude AIHA; in one series of 100 children with AIHA, DAT was negative in 21%.25
Common DAT findings associated with different AIHAs.1 13 AIHA, autoimmune haemolytic anaemia; CHAD, cold haemagglutinin disease; PCH, paroxysmal cold haemoglobinuria.
In PCH, the D-L bound RBCs are mostly coated with C3 causing the DAT-positive for complement (C3) (DAT-positive C3) screen. Children presenting with features suggestive of intravascular haemolysis and have a DAT-positive C3 should undergo an indirect D-L test for PCH.1 A DAT-negative test does not exclude PCH; one study estimated that 1 in six children with PCH were DAT-negative.19 Consequently, a D-L test should equally be considered in children with haemolysis, haemoglobinuria and DAT-negative.1
What is the indirect D-L test and are there any problems with the test in a child undergoing the D-L test?
The indirect D-L test subjects the patient’s serum to appropriate cells, P-antigen-positive group O RBC and fresh donor serum (a source of complement) and incubates at 4°C followed by 37°C. Presence of the D-L antibody is confirmed by the initiation of haemolysis postsuccessive incubations. If no haemolysis occurs, theoretically the D-L antibody is absent (or a false-negative result). Figure 3 shows the positive D-L test of the patient and gives further information on the D-L test. The test takes approximately 24 hours in a weekday and 72hours on a weekend and may need performing at a specialist immunohaematology centre, incurring delays if transport is limited. The lab will appreciate forewarning, and the test costs approximately £108.
The most common cause of false-negative D-L tests are low and undetectable D-L antibody titres.26 This can be due to the rate at which the D-L antibody disappears from plasma during patient recovery.26 Using fresh donor serum as the source of complement and maintaining the patient’s serum sample at 37°C should reduce the incidence of false-negative tests. False-positive results have occurred in cases where a patient has CHAD and IgM autoantibodies.27 More accurate estimation of the sensitivity and specificity of the D-L test is limited by technical issues (transport to a regional haematology lab given the temperature requirements) and infrequent use.
How does a positive D-L test and subsequent diagnosis of PCH influence management of a child who presented with an acute haemolysis?
Management of PCH has primarily been supportive: cold avoidance, maintaining a warm ambient temperature (prewarming all intravenous fluids and transfusions), giving transfusions as required and folic acid supplementation to avoid megaloblastosis.1 4 15 16 Simultaneous treatment of relevant underlying causes should be undertaken, for example, persistent bacterial infections or syphilis. Corticosteroids have previously been used, although there is significant uncertainty regarding efficacy due to the transient nature of haemolysis.4 10 27 28 Children tend to be treated with corticosteroids initially (prednisolone 1 mg/kg daily like in warm AIHA), as a precautionary step.29 Once PCH has been diagnosed, corticosteroids can be stopped with the exception of severe or refractory disease where a 1-week trial of corticosteroids would be considered.1 25 Other medications considered in AIHA can also be avoided with a PCH diagnosis including immunosuppressants (rituximab).1 For refractory, chronic or recurrent PCH case reports for immunosuppressive therapies (rituximab, intravenous IgG or eculizumab (humanised anti-C5 monoclonal antibody)) exist, but robust trials are needed.4 30
PCH is usually self-limiting and tends to resolve spontaneously when the viral illness or trigger has subsided.14 15 As an intravascular haemolysis, it can rapidly become life-threatening with a severe progressive anaemia and acute kidney injury secondary to haem pigment nephropathy.31 Renal function must be monitored frequently; adequate renal perfusion must be maintained; and nephrotoxic drugs, for example, NSAID-based antipyretics, must be avoided.31
Transfusions to maintain Hb at a clinically acceptable level are indicated when a child develops hypoxaemia, cardiac decompensation or a rapidly progressive anaemia.6 Children tend to compensate better for a falling Hb and transfusions are usually given when Hb is <5–6 g/L (above this consider child’s clinical status).29 The laboratory must be informed of the diagnosis so that pretransfusion testing can be performed above the thermal amplitude of the D-L antibody to avoid agglutination and delays to transfusions.
To monitor disease progression and recovery regular blood tests for Hb, reticulocytes, bilirubin and LDH should be done.29 An increase in reticulocytes, usually within 3–5 days, is an important marker of recovery in haemolysis.5 Likewise, a decrease in LDH corresponds with a reduction in the haemolytic rate.5 After initial recovery, monthly blood tests would be reasonable and can be discontinued after two consecutive normal results.29
With the reduced incidence of congenital syphilis, PCH is no longer viewed as a chronic disorder, and characteristically, once resolved it rarely reoccurs with long-term prognosis being favourable.13 16 The family must be counselled on how to recognise future episodes of AIHA as, although rare, reports have highlighted PCH recurrence up to 21 months after initial presentation.32 Where PCH persists or resolution is slow, a paediatric haematology opinion may help in looking for rarer causes,for example, lymphoproliferative disorders.29
Management: The patient was initially treated with folic acid, steroids, blood transfusions and intravenous ceftriaxone to cover for any bacterial infections. Due to vascular compromise, he required a femoral line. We maintained a warm environment, minimised exposure to cold and gave fluids and blood through a warmer. A positive D-L test confirmed PCH and the steroids were stopped. After 10 days, he was discharged home once haemodynamically stable and his Hb showed a consistent upward trend. We continued to monitor his recovery for 4 weeks as an outpatient until his reticulocytosis had resolved (reticulocytes=62×109/L); Hb returned to the normal level (Hb=128 g/L); and there was no further evidence of haemolysis. The patient did develop a thrombus in his femoral line and this was successfully treated with Dalteparin alongside his PCH. Advice was given to the family on recognising the clinical signs of acute haemolysis and when to seek medical attention should any further episodes occur.
Conclusion
PCH is a rare but one of the most common causes of AIHA in children. We demonstrate how the use of the D-L test diagnosed our 2-year-old boy with PCH and helped with subsequent management and avoiding unnecessary treatments, for example, steroids and immunosuppressants. It is reasonable to perform the D-L test in children with evidence of intravascular haemolysis and/or a DAT-positive C3 test. From a paediatrician’s perspective, ensuring the laboratory is forewarned and keeping the sample at 37°C help maximise the quantity of antibody in the serum and the likelihood of performing a D-L test correctly.
Topics for further research
The role of the D-L test in DAT-negative AIHAs.
Sensitivity and specificity of the D-L test.
Test your knowledge
What are the typical laboratory findings in patients with intravascular haemolysis?
Raised bilirubin, raised haptoglobin, low haemoglobin (Hb), raised lactate dehydrogenase (LDH).
Raised bilirubin, low haptoglobin, low Hb, raised LDH, haemoglobinuria.
Low bilirubin, low haptoglobin, low Hb, raised LDH, haemoglobinuria.
Low bilirubin, raised haptoglobin, low Hb, raised LDH.
Direct antiglobulin test (DAT) testing in paroxysmal cold haemoglobinuria (PCH) is likely to reveal the following:
DAT testing: IgG positive, C3 negative.
DAT testing: IgG negative, C3 negative.
DAT testing: IgG negative or positive, C3 positive.
Donath-Landsteiner (D-L) antibody is classically a biphasic haemolysin. What does this mean with regards to PCH?
D-L antibody fixes complement to red blood cells (RBCs) at low temperatures (<37°C) and haemolysis is triggered when the blood is re-warmed to 37°C.
D-L antibody fixes complement to RBCs at 37°C and triggers haemolysis.
D-L antibody fixes complement to RBCs at low temperatures (<37°C) and triggers haemolysis.
D-L antibody fixes complement to RBCs at 37°C and haemolysis is triggered when the blood is cooled to 4°C.
There is little evidence to suggest that steroids are effective in the management of PCH. True or false?
Performing the D-L test is difficult. What must clinicians remember to do if they want the test to be successful?
Maintain temperature of the serum sample at 37°C until the test is carried out.
Take the blood test in the evening.
Maintain temperature of the serum sample at 4°C until the test has been carried out.
Doesn’t matter what temperature the serum sample is kept at on transfer to the lab.
Answers to the quiz are at the end of the references
Answers to the multiple choice questions
B
C
A
True
A
Supplemental material
Ethics statements
Patient consent for publication
Acknowledgments
We are grateful for the information and assistance of the Poole Pathology and Severn Pathology services in the preparation of this article.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors JDW, HK and MPT conceived the article. JDW wrote the article, with assistance from HK and MPT, and edited the article for publication. RKJ gave haematological advice. All authors are accountable for this work.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.