Article Text
Statistics from Altmetric.com
Background
It has been known for centuries that fasting can suppress seizure activity in patients with epilepsy.1 In 1921, it was suggested that a ketogenic diet, designed to induce and sustain the metabolic effects of fasting, might have the same beneficial effects.2 This diet, high in fat and low in carbohydrates and proteins, was popular until the introduction of anticonvulsants such as phenytoin.3 In the 1990s, interest in the diet was renewed, following its successful use to treat a child with drug-resistant epilepsy, and the establishment of an awareness raising support group by the father of the child.3
This article provides a brief overview of ketogenic diets and then focuses on the issues relating to the use of medicines in patients on these diets.
When is dietary therapy suitable?
Approximately 25% of children with epilepsy have an inadequate response to antiepileptic drugs.4 Consideration of ketogenic diet use has been recommended under the following circumstances:
In children who have failed 2–3 anticonvulsant therapies, regardless of age and gender, and particularly in those with symptomatic generalised epilepsies (International Ketogenic Diet Study Group (IKDSG), 2008).5
In children and young people with epilepsy whose seizures have not responded to appropriate antiepileptic drugs (AEDs) and who have been referred to a tertiary paediatric epilepsy specialist (National Institute for Health and Care Excellence guidance, 2012).6
Contraindications to the ketogenic diet include pyruvate carboxylase deficiency, β-oxidation defects, primary carnitine deficiency and porphyria.3
What are the different types of ketogenic therapy and how do they differ?
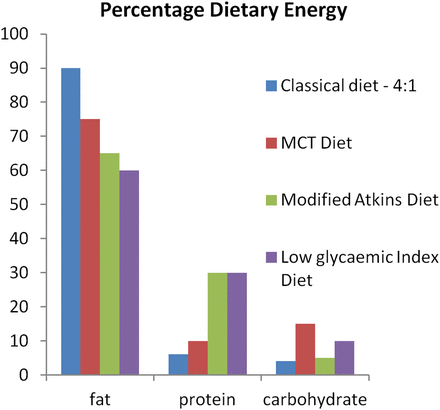
Currently, four types of ketogenic therapy are in use: the classical ketogenic diet, the medium-chain triglyceride (MCT) diet, the modified Atkins diet (MAD) and the low glycaemic index (LGI) treatment. For the classical ketogenic diet, the ratio of fat to carbohydrate and protein ranges from 2:1 to 4:1 and it is predominantly long-chain fat sources that are used. As the name suggests, the MCT diet derives most of its fat from medium-chain fat sources, which are more effective at inducing ketosis than long-chain fat sources.5 Further differences between the four diets are shown in figure 1.
Macronutrient content of ketogenic therapies (adapted from Zupec-Kania et al 7). MCT, medium-chain triglyceride.
Numerous studies have looked at the efficacy of the classical ketogenic diet in children with refractory epilepsy. Many of these studies were uncontrolled and with considerable variation in study design, but analysis has produced consistent results: approximately half of all children achieve >50% reduction in seizure frequency and approximately 10%–15% of children become completely seizure free.3 ,4 ,7 Existing evidence is limited, but suggests that similar results are achieved with the other ketogenic diets.7 The choice of an appropriate diet is therefore made on an individual patient basis, with the child's age, family circumstances, and the type and severity of epilepsy all being taken into account. Factors such as the speed of response required and the availability of an appropriately experienced and supported paediatric dietitian at the treatment centre should also be considered.4
The first three months may be a critical period in the dietary therapy of epilepsy. For the MAD and classical diets, there is limited evidence that the key to efficacy is taking a strict approach during this initial period,4 that is, using a MAD with a high fat content or low carbohydrate content, or a classical diet with a lipid:non-lipid ratio of 4:1.4 ,8
How does ketogenic therapy work?
Many researchers believe that ketosis is not the primary way ketogenic diets work but rather the metabolic shift that occurs with the treatment.3 In a recent review, evidence for four possible mechanisms was summarised: carbohydrate reduction (2-deoxy-d-glucose, a glucose analogue, partially inhibits glycolysis and has demonstrated antiseizure activity in animals), activation of ATP-sensitive potassium channels by mitochondrial metabolism, inhibition of glutamatergic excitatory synaptic transmission and inhibition of the mammalian target of rapamycin pathway (overactivation of the mammalian target of rapamycin might cause seizures by promoting epileptogenesis).9
What are the adverse effects of ketogenic therapy?
Short-term adverse effects seen when a ketogenic diet is started include vomiting, acidosis, constipation, worsening of gastro-oesophageal reflux disease (GORD), excessive ketosis, food refusal, fatigue, exacerbation of seizures and hypoglycaemia.10 The ketogenic diet lacks fibre and bulk, leading to gastrointestinal problems.11 GORD is common because fat lowers the oesophageal sphincter tone, slows gastric emptying and decreases intestinal transit time. Fluid restriction worsens symptoms and ketone bodies induce anorexia or feeding refusal.11
Long-term use of ketogenic therapy is often associated with vitamin D deficiency; there may be decreased bone mineral density and an increased risk of bone fractures.10 Growth disturbance is also common after 6–12 years.10 Dyslipidaemia is seen in around 60% of patients on the classical diet,10 but there is no evidence that it is a long-term adverse effect.12 Kidney stones have been reported in 10%–25% of patients on the ketogenic diet.10 ,11 Secondary carnitine deficiency occurs only rarely.10 Cardiac abnormalities have been reported in case studies though there has been no systematic evaluation of the cardiovascular risks of the ketogenic diet or assessment of coronary arteries of adults who were on ketogenic diet as children.11 In a retrospective chart review of patients who had been on the diet for >6 years, 24 out of 28 patients had experienced more than a 90% reduction in seizures. Also, 23 were <10th centile for height at the most recent follow-up compared with 10 at initiation of ketogenic diet (p=0.001). Kidney stones had occurred in seven and skeletal fractures in six patients. The authors recommend that growth, kidney stones and fractures should be monitored closely.12
Hyperlipidaemia occurring during dietary therapy can be treated with fat ratio or composition changes to the diet, weight loss or weight gain by adjusting calorie intake.3 Adverse effects such as acidosis and constipation are also treatable, but attention is now turning to the prevention of adverse effects before they occur, and one method of preventing these effects may be the routine use of supplements.3 As with medicines, supplements with a low or no carbohydrate content are preferable (see later). Table 1 lists supplements recommended by members of the IKDSG.5 Some are universally recommended, whereas others are optional.
Supplementation for children on the ketogenic diet (adapted from Kossoff et al 5)
Children initiated on the ketogenic diet should undergo regular blood monitoring, urinalysis and growth monitoring.14 They generally attend a multidisciplinary clinic three-monthly during the first year and six-monthly thereafter, with younger children and those with feeding or other issues attending more frequently.11
Further research is required to determine whether the more liberal diets are associated with a milder adverse effect profile. There is limited evidence for this.16 ,17
What are the implications for use of medicines?
Carbohydrate content of medicines
Medications taken by children are often suspensions or solutions with high carbohydrate content. By preventing the initiation of unnecessary high-carbohydrate medicines and recommending the use of low/no carbohydrate formulations/preparations whenever appropriate, dieticians and pharmacists can reduce the amount of carbohydrate ingested by children on ketogenic diets in their medicines, thereby enabling them to maximise their dietary carbohydrate intake.
In general, medicines with a carbohydrate content of <1 g per dose are not taken into account when the carbohydrate content of the diet is calculated and this has been proposed as the goal for each dose of medicine.18 However, many patients have a daily carbohydrate intake between 12 and 20 g per day, so if the patient is on multiple medicines with frequent daily doses, drugs can take a significant portion of the daily calories. If the ketosis is unstable or low, a change to a formulation with a lower carbohydrate content or glycaemic load should be sought.
A case report describes a 10-year-old patient with myoclonic astatic epilepsy (Doose syndrome) who had been well controlled on ketogenic diet, rufinamide and clobazam. She presented with multiple breakthrough seizures, and it was established that she had been started on amoxicillin five days earlier. Review of the tablets being used revealed a high carbohydrate content and she had markedly decreased β-hydroxybutyrate levels from baseline. A switch to a different formulation resulted in seizure remission the next day.19
There is no single source available in the UK for checking the carbohydrate content of medicines, and up-to-date information on this should be sought before decisions on medication changes or initiation are taken.
Information from the Charlie Foundation website has been adapted to produce table 2.14 ,20 When considering the excipients in a medicine, the glycaemic index (GI) and energy provision of the carbohydrate should be taken into account. Medicines with excipients with a higher energy value/GI should be avoided preferentially (see table 3).
Ingredients to be considered in ketogenic diets
Comparison of sugar and sugar alcohols (adapted from Livesey21)
General guidance:
Tablets usually contain less carbohydrate than liquids.22
Suppositories can be used as any carbohydrate they contain is not absorbed.22
Avoid syrups, elixirs and chewable tablets, if possible.14
Sugar-free preparations often contain sorbitol, which should be minimised, if possible,14 as should maltitol.
When treating constipation, oral macrogols and phosphate enemas can be used.22 Lactulose and senna are often avoided.22 Although there is no evidence that the carbohydrate in lactulose is absorbed, the carbohydrate load is high, therefore avoidance is cautionary.
Avoid intravenous fluids containing glucose unless the child has low blood glucose levels.22
If a new medicine is started, monitor ketones and contact the ketogenic team if there is concern that the medicine is causing a reduction in ketones.22
When a child is admitted to hospital, stickers on the drug chart,22 venous lines or bed, and electronic warnings on the hospital system can highlight the fact that the patient is following a ketogenic diet and remind staff that new medications should be assessed for carbohydrate content. Combining the use of such alert systems with relevant staff education programmes may reinforce the message.
The carbohydrate content of any non-prescription medicines and supplements that the patient is taking should also be considered.
Ideally, medication changes are avoided during the initiation and ‘fine tuning’ stages of a ketogenic diet.14 Strict adherence to the diet during the first one to three months could be important for efficacy,4 but may not be possible. For instance, acute emergency seizure treatment for prolonged seizures and seizure clusters may be required and should be given.14
When ill, children should be treated appropriately for illness and medications in their lowest carbohydrate form used whenever possible. Blood sugars should be checked two to four hourly and corrected appropriately. Ketones should also be monitored. It may be necessary to stop the ketogenic diet temporarily.10 ,14
Medicines and the ketogenic diet
Children who start on the ketogenic diet are often already taking at least one anticonvulsant as well as various other medications. As well as determining the carbohydrate content of these medications, it is important to consider the potential of the diet to affect drug serum levels and effects. Surprisingly, despite decades of combined use, it remains unclear whether there are negative or positive pharmacodynamic interactions and only scant information regarding the impact of ketogenic diets on the pharmacokinetics of anticonvulsants.
The IKDSG states that the ketogenic diet is not negatively affected with regard to efficacy or adverse effects by any particular anticonvulsant.5 In a recent review, however, phenobarbital was listed as a relative contraindication.3 In a retrospective cohort study including 115 children, those on phenobarbital in combination with the ketogenic diet were significantly less likely to have >50% seizure reduction (p=0.003) than those combining the diet with other anticonvulsants. Conversely, those on zonisamide at onset of the ketogenic diet were more likely to have >50% reduction in seizures (p=0.04).23
There is a historical perception that valproate should not be used with the ketogenic diet. This stems from concerns for idiosyncratic reactions such as hepatotoxicity and given the fact that it is a short-chain fatty acid. Despite these fears, clinical evidence supports the safe use of the combination.5
Absorption issues
The ketogenic diet can cause vomiting in some children, potentially leading to incomplete absorption of anticonvulsants. One author suggests that in some cases it may be appropriate to give a further dose when vomiting has occurred depending on the timing of vomiting in relation to medicine intake and other factors such as drug pharmacokinetics and formulation.7
Slowed gastric emptying can lead to delayed absorption of anticonvulsants and reduced seizure control. Consideration could be given to dosing more frequently with smaller doses;7 however, this has practical implications for parents and carers.
Protein binding and metabolic effects
Since protein intake is reduced in children receiving ketogenic therapy, levels of drugs that are protein bound might be expected to alter. Transient increases in serum levels of AEDs have been reported on ketogenic diet initiation but these seem to stabilise.7 When serum levels of valproic acid, lamotrigine, topiramate, clonazepam and phenobarbital were measured immediately before and three months after initiation of the ketogenic diet, no statistically significant differences were found between pre and during diet levels.24
Valproic acid has a ketone metabolite so can cause false-positive urinary ketone tests in patients on a ketogenic diet.7 Secondary carnitine deficiency, which can occur with the ketogenic diet or with valproic acid, has been reported to be exacerbated in children on both.5 ,25
It has been suggested that acidosis peaks on initiation of the diet.10 This is worth noting since central nervous system drug levels can be affected by plasma pH changes and drug elimination by urinary pH changes.7
Carbonic anhydrase inhibitors such as topiramate and zonisamide can also cause metabolic acidosis and kidney stones.7 The IKDSG recommends monitoring bicarbonate levels in patients receiving topiramate and zonisamide, and giving bicarbonate supplements if there are symptoms of acidosis, for example, vomiting, lethargy.
Discontinuation of medicines
Once established on a ketogenic diet, many children are able to discontinue anticonvulsants. Although it is generally advised that this is not attempted until a child has been treated successfully with the diet for several months, there is some evidence that anticonvulsants can be reduced with good results during the first month.5 Exacerbation of seizures is more likely to be associated with the gradual discontinuation of phenobarbital or benzodiazepines than with other anticonvulsants.5
When and how should the ketogenic diet be discontinued?
Parents are counselled to continue the ketogenic diet for at least three months even if apparently ineffective. Recent data suggest that it works rapidly when effective, with 75% of children responding within 14 days. Expert opinion is that the diet should be followed for a mean of 3.5 months before discontinuation is considered. However, if seizures worsen for more than a few days after starting the diet, immediate discontinuation is justifiable.5 In children experiencing a reduction in seizures of >50%, the diet is usually continued for about 2 years, but treatment for up to 12 years has been found to be helpful in children with few side effects in whom seizures are greatly reduced, for example, >90%.5 For those in whom the diet has led to freedom from seizures, 80% will remain seizure free after the diet is discontinued.
Usually, discontinuation involves reducing the ketogenic ratio over a few months, for example, from 4:1 to 3:1 to 2:1, perhaps with reductions being made every two weeks, until urinary ketosis is lost.5 ,7 A cautious reintroduction of carbohydrate-rich foods can then occur.7 If there is an increase in seizures, the ketogenic ratio can be increased to the last effective ratio.5
Once the diet is established, abrupt discontinuation, other than when children are hospital inpatients, is best avoided as it can lead to worsening of seizures.10
Acknowledgments
This article was adapted from a review commissioned and written for the Neonatal and Paediatric Pharmacists Group.
References
Footnotes
Contributors RM planned this article and was involved with editing and finalising it. AB performed the literature searches and undertook much of the writing and final editing. HC provided valuable dietetic input.
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.