Article Text
Statistics from Altmetric.com
Answers TO THE EPILOGUE QUIZ
From questions on page 144
The answers to question 1 are C, E, H, I, J.
Three broad categories of aetiologies can be considered for his renal impairment.
-
NSAID use.
-
Prerenal impairment secondary to dehydration, which should be readily reversible with volume replacement. Volume depletion alone can cause acute tubular necrosis.
-
Infectious. Any patient returning from a tropical country with fever should have imported tropical illnesses high on the differential list. The activities of swimming in open water and eating partially cooked meat increased the likelihood of this patient having an imported tropical infective cause. Renal involvement is a recognised feature of a wide number of tropical illnesses, including leptospirosis, malaria, typhoid, viral haemorrhagic fever and HIV.1 It may present as a PIGN, haemolytic uraemic syndrome (HUS), or with prerenal failure secondary to the diarrhoeal element.
In the case above, a recent outbreak of dengue had occurred in the region, prompting this as a possible diagnosis. Information on local bacterial epidemiology can be useful in pointing towards a diagnosis. Considering the options above, malaria would be unlikely with negative films and clear urinalysis, HUS is excluded by the normal haemoglobin and platelets, PIGN again by the clear urinalysis. Rickettsia does not typically cause any renal impairment. Salmonella, while plausibly causing renal impairment through hypovolaemia alone, also causes a rhabdomyolysis-induced renal impairment, which is not the clinical picture here given the normal serum creatine kinase and absence of myoglobin/haemoglobin on urinalysis. Dengue, leptospirosis and typhoid could all be possible infectious agents.
The answers to question 2 are pyrexia, vomiting, diarrhoea, NSAID use
Risk factors for NSAID-induced toxicity are those that cause hypovolaemia. In this case, the high fever would contribute to higher insensible losses, while diarrhoea and vomiting deplete total body fluid. Ibuprofen and other NSAIDs may precipitate renal impairment in dehydrated patients.2 Unfortunately, hot vomiting small children are exactly the kind of patients that commonly receive NSAIDs!
NSAIDs inhibit prostaglandin-mediated vasodilation. Local release of prostaglandins is a compensatory mechanism in cases of reduced blood flow to the kidney (such as in dehydration and hypovolaemia), to ensure an adequate glomerular filtration. Removing the ability of a potentially compromised kidney to compensate can rapidly lead to significant impairment. In addition, NSAIDs have a direct toxic effect on renal tissues, which can manifest as acute tubular necrosis, interstitial nephritis or rarely papillary necrosis.3
There is a dose-dependent effect of NSAIDs. The risk of renal impairment increases with greater doses, and with less selective non-steroids.
Answer to question 3

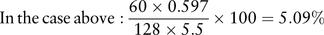 FeNa can be useful in narrowing the differential diagnosis. A low FeNa (<1%) suggests avid retention of sodium in the context of dehydration or hypovolaemia, in an attempt to improve intravascular volume. Acute tubular necrosis leads to inappropriate sodium wasting due to failure of tubular reabsorption, with a resulting FeNa of >2–3%.
FeNa can be useful in narrowing the differential diagnosis. A low FeNa (<1%) suggests avid retention of sodium in the context of dehydration or hypovolaemia, in an attempt to improve intravascular volume. Acute tubular necrosis leads to inappropriate sodium wasting due to failure of tubular reabsorption, with a resulting FeNa of >2–3%.
Such a high FeNa in this case indicates that the renal impairment cannot be explained by hypovolaemia and dehydration alone. NSAIDs may cause acute tubular necrosis and salt wasting, though more typically they lead to sodium retention. Leptospirosis preferentially affects the kidney as a site of infection, and typically causes a tubulopathy, with sodium wasting a common feature.4 Dengue haemorrhagic fever frequently leads to acute kidney injury, while classic dengue is associated with proteinuria as the main finding though various renal presentations have been described. Tubular involvement and salt wasting is rare, however. Typhoid fever may cause HUS or rhabdomyolysis-related kidney injury; again, isolated tubular leak is rare.
Further clinical information
Infectious disease diagnostics were sent, all of which were negative except for a positive leptospirosis IgM titre 1:160 by ELISA. The microscopic agglutination test for leptospirosis was negative to all serogroups, as was PCR for leptospirosis DNA. This was presumed due to a combination of ongoing antibiotic therapy and resolution of the bacteraemia. The biphasic nature of the illness with symptomatic improvement and then worsening is very typical of infection with leptospirosis (though it is also seen in dengue). A repeat serology in the convalescent phase demonstrated persistent positive leptospirosis IgM (titre 1:80) confirming the diagnosis.
The combination of mild elevation of transaminases, characteristic fever with headache and myalgia, short-lived rash, and biphasic symptomatology with apparent improvement would all be very consistent with leptospirosis, though also commonly seen in dengue. Jaundice is common in patients severely afflicted by leptospirosis. There is an increasing recognition that many patients are asymptomatic or have mild disease only. Anicteric leptospirosis is classically less severe and not typically associated with renal impairment, while the development of jaundice indicates more likely progression to renal failure and haemorrhage, with the potential for multiorgan failure, ‘Weil's syndrome’. Renal impairment is recognised to occur without jaundice,5 and prompts diagnostic testing in endemic regions.6 ,7 However, leptospirosis is an unusual pathogen in the UK. Less than 70 cases occurred in the UK in 20128 with a third being acquired abroad. Renal impairment was identified in 26% of those cases. The absence of jaundice may be more common in imported disease, occurring in 50% in a German cohort.9 Renal impairment in leptospirosis is typically hypokalaemic rather than hyperkalaemic, presumed due to an aldosterone effect.10 It is also usually non-oliguric, whereas here the polyuric phase followed the administration of intravenous fluids.11 ,12 Finally, the absence of any haematuria or casts on microscopy is also unusual in leptospirosis.
Treatment of leptospirosis may be with intravenous benzylpenicillin or a cephalosporin in severe illness, or oral doxycycline, ampicillin or amoxicillin. Currently there is insufficient evidence available to advocate for or against antibiotic therapy for leptospirosis.13
Though a final diagnosis cannot be definitive, we suspect this patient had renal injury from leptospirosis infection and NSAID-associated toxicity. The patient made a full recovery.
References
Footnotes
-
Contributors All authors were involved in the clinical care of the patient. BCR prepared the article, and all other authors provided critical revisions. All authors approved the final version.
-
Competing interests None.
-
Patient consent Obtained.
-
Provenance and peer review Not commissioned; internally peer reviewed.


