Article Text
Abstract
Adequate nutrition and growth during the neonatal period are important, especially for preterm infants, for whom there is evidence of poor nutrient intakes and growth, and this has important implications for their health in later life. Increased nutritional support while on the neonatal intensive care unit has been shown to improve growth, but such support is not universally available. Being able to carry out and interpret a nutritional assessment is therefore an important skill for paediatricians caring for neonates. This article aims to explain how to use nutritional assessment in neonates and provides some tools to make this process as straightforward as possible.
- Nutrition
- Neonatology
- Growth
Statistics from Altmetric.com
Introduction
Preterm infants often experience poor growth while in the neonatal intensive care unit (NICU), resulting in them being discharged with a weight, length and head circumference on growth chart centiles substantially lower than those on which they were born.1 The causes for this poor growth are multifactorial; in addition to respiratory and metabolic instability after birth and concurrent illnesses during the neonatal period, gastrointestinal immaturity and the increased nutrient needs of growing preterm infants make the provision of adequate nutrition a challenge. Inadequate nutrient delivery and variability in nutritional care are well described in the literature and correlate with the degree of growth failure seen.2 ,3 Increased intakes of energy and protein in the first week of life (regardless of growth) have been associated with improvements in neurodevelopmental outcomes at 18 months of age.4
Provision of increased nutrition support is an effective strategy to address nutritional deficits in the NICU,5 and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) recommend the implementation of specialist paediatric nutrition support teams in hospital.6 However, as this resource is not consistently available, the ability to undertake nutritional assessments of infants as part of routine care and use the findings to inform management represents an important skill for paediatricians caring for neonates, and provides an opportunity to improve growth outcomes during hospital stay. This article explains how to carry out nutritional assessments in neonatal patients.
Physiological background
The purpose of nutritional assessment is to document objective nutritional parameters, identify nutritional deficits and establish nutritional needs for an individual patient. Identifying poor growth and shortfalls in nutrient intake allows them to be addressed with the aim of optimising nutritional status and improving growth and later outcomes.
Growth reference standards and growth curves
Growth charts based on reference standards are used to assess adequacy of growth in children. In the UK since 2009, the growth reference used for preterm and unwell neonates is the UK-WHO Neonatal and Infant Close Monitoring growth chart, which incorporates longitudinal WHO growth data for children from 2 weeks to 4 years of age, but uses older, cross-sectional birthweight data from the UK 1990 dataset for preterm infants from 23 weeks gestation until 42 weeks. There is debate about the utility of cross-sectional birthweight data to approximate intrauterine growth; the birth weights of preterm infants may not represent ‘normal’ intrauterine growth and intrauterine growth may not be an appropriate postnatal target for preterm infants given the differences between the in-utero and ex-utero environments. A true ‘growth chart’ using longitudinal data from preterm infants followed up and measured over time may be preferable, but such a standard would depict ‘actual’ rather than ‘ideal’ growth, especially in the context of preterm infants receiving suboptimal nutrition. Cole et al7 recently published weight centiles for preterm infants born below 32 weeks gestation based on longitudinal UK data, which demonstrated this, with infants’ mean weight falling at least two marked centile lines in the first 2 weeks of life.
While what constitutes ‘ideal’ growth for preterm infants remains unclear, regardless of the growth standard used, the utility of growth charts is to monitor an infant's growth over time in relation to marked centiles. Although immediately after birth growth may be slow, with infants crossing down 2–3 marked centile lines, from the second or third week of life infants should be growing consistently along, or parallel to, a centile line, as demonstrated by the data of Cole et al.7 Consistent downward crossing of centiles after the third week of life should be considered growth failure, while crossing up centiles may represent catch up growth (providing it occurs proportionally across all parameters). Increases in weight in the absence of gains in length and head circumference may represent excess relative adiposity due to a failure to gain lean mass.8
Recommended nutrient intakes
Recommendations for the nutrient intakes of term infants have been derived based on the assumption that breast milk was the ideal feed for term infants. Using the composition and volume of breast milk taken by term infants growing in accordance with contemporary reference standards, it was possible to calculate reference nutrient intakes (RNIs) (see table 1 and figure 1) for term infants (table 2).9 It is not possible to use the same method to derive preterm infant requirements, as such assumptions cannot be made and ‘ideal’ growth has yet to be adequately defined. Nonetheless, published recommendations for the nutrient intakes of preterm infants are available, with the most widely used being those in ‘Nutrition of the Preterm Infant: Scientific Basis and Practical Guidelines’ originally by Tsang et al,10 and recently updated by Koletzko et al.11 This provides recommendations for infants <1500 g (table 2). Similar recommendations were produced in 2010 by the ESPGHAN for infants <1800 g at birth12 and by the WHO for infants weighing <2500 g (table 2).13 Such recommendations have been derived from fetal composition studies, nutrient balance studies, clinical trials, umbilical cord blood nutrient levels and extrapolated term infant data. Consensus was used in the absence of robust evidence and recommendations for each nutrient given as a ‘reasonable range of intake’ (table 1), rather than RNI, in recognition of this. While there is debate as to the validity of these recommendations, there is evidence that achieving them can improve growth.14
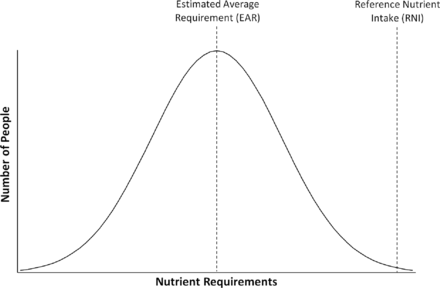
Terminology for nutrient intakes
Estimated average requirement for intake of a nutrient and reference nutrient intakes displayed in relation to the requirement of a given population.
Nutritional requirements of neonates (for fully enterally fed infants, amounts are per kg per day unless otherwise stated)
Technological background: carrying out a nutritional assessment
Inadequate nutrition is often not obvious and its effects not immediate. Consequently, nutrition is often overlooked, with more acute issues such as respiratory or cardiovascular disease justifiably taking precedence. It is only by actively undertaking regular nutritional assessments that nutritional problems can be identified and addressed. A weekly ‘nutrition round’ on the NICU is a good way of incorporating such assessments into clinical practice. An ‘ABCDE’ approach to nutritional assessment (box 1) can be used to keep things clear, as detailed below.
ABCDE approach to nutritional assessment (note laboratory reference values may vary between hospitals)
Anthropometry
–Weight
–Head circumference
–Length
–Plot measurements on growth chart
Biochemistry
–Serum glucose
–Serum triglycerides (should be <2.8 mmol/L if tolerating parenteral lipid)
–Serum electrolytes (to enable adjustment of PN)
–Urea <1.6 mmol/L may indicate inadequate protein intake
–Bone markers: serum phosphate levels a good reflection of intake, and phosphate <1.8 mmol/L and ALP >900 IU/L may indicate metabolic bone disease
–Vitamins and trace elements useful if on long term PN
Clinical assessment
–Hydration status, oedema and fluid needs
–General health
–Diseases that may affect nutritional requirements or tolerance/absorption of nutrition
Dietary assessment
–Calculate intakes of energy and protein (and any other nutrients of interest) from parenteral and enteral intakes
–Compare intakes with recommended amounts
Evaluation
–Take into account findings from all of above and decide if intakes and growth are adequate
–Formulate plan to address shortfalls or excesses
ALP, alkaline phosphatase; PN, parenteral nutrition.
A—anthropometry
Anthropometry, specifically regular measurement of weight, length and head circumference, is one of the mainstays of nutritional assessment. Most infants on the NICU will be weighed at least twice weekly, with head circumferences and length ideally measured weekly. This is essential for the correct interpretation of trends in weight in the context of overall growth. Measuring rods built into ward scales (figure 2A) allow length to be conveniently measured alongside weight, negating the need to use more cumbersome ‘infantometers’ (specific devices for measuring the length of supine infants, figure 2B). Newer ‘incubator measures’ (figure 2C) allow length measurement of preterm infants while in their incubator. It is important to consider both the quantity and quality of growth, and plotting all measures on an appropriate growth chart allows assessment of whether an infant is growing adequately and in proportion. Quality of growth can also be assessed by measuring an infant's body composition (their relative proportions of fat and lean tissue), and there is evidence that the pattern of body composition achieved by preterm infants by term equivalent age is different from that of term born infants.8 There are several methods which are valid and feasible for use in neonates and these are covered in detail elsewhere.15 However, all require specialist equipment and are subject to limitations, with no practical and reliable method of assessing body composition during the NICU stay currently available. There is a need to develop such techniques to help guide nutritional care.
Measuring devices for neonates ((A) scales with built in measuring rod, (B) infantometer, (C) incubator measure).
B—biochemistry
Certain biochemical parameters can provide a useful insight into nutritional status while others are less useful, as detailed below.
Glucose and lipids: Hyperglycaemia (blood glucose >10 mmol/L16) is common in preterm infants after birth and can necessitate a reduction in the glucose content of parenteral nutrition (PN) or the commencement of insulin. Similarly, serum triglyceride levels measured during infusion will indicate tolerance of intravenous lipid, with a level >2.8 mmol/L suggesting a need to reduce PN lipid content.17 Both hyperglycaemia and hyperlipidaemia are important; subsequent reduction in the carbohydrate and lipid content of PN will limit the ability to provide adequate protein, as an energy to protein ratio of around 20–25 non-protein kcal per gram of protein11 should be maintained in order to provide enough energy to enable protein to be used for tissue accretion and growth. Inadequate energy to protein ratio or increased catabolism (e.g. sepsis or surgery) preventing growth results in unused excess nutrients that must be excreted, adding to renal solute load and increasing metabolic demand. It is therefore important to ensure adequate energy to protein ratios and consider avoiding excessive nutrient intakes during periods of catabolism.
Markers of protein status: Serum total protein levels are not related to intake, as the levels of many proteins, particularly acute phase proteins such as C reactive protein and ferritin, are independent of nutritional status. Albumin has a long half-life (around 20 days) and so cannot be used to monitor protein status in the short term. While serum urea cannot accurately predict protein intake, levels of <1.6 mmol/L suggest a protein intake of <3 g/kg/day.18 Protein intakes exceeding an infant's metabolic capacity (likely >5 g/kg/day) could potentially lead to high plasma amino acids levels (including tyrosine and phenylalanine, which may affect mental development).19
Electrolytes: Serum sodium and potassium are poor indicators of total body stores due to homeostatic mechanisms acting to maintain normal serum levels despite changes in supply and demand. However, excessively low or high levels can be useful in determining the requirement for these electrolytes in feeds and fluids. Preterm infants have limited ability to concentrate their urine, and so high urinary sodium and potassium levels (>30 mmol/l) can be normal. In this context, a urinary sodium <10 mmol/L may indicate insufficient intake, especially in the presence of relevant pathology such as high ileostomy losses.10
Markers of bone status: Like sodium and potassium, serum calcium is a poor marker of body stores. Serum phosphate does fluctuate in response to intake, with low levels indicating the need for supplementation. Chronic phosphate deficiency increases the risk of metabolic bone disease by upregulating 1,25(OH)2 vitamin D production, increasing osteoclast activity and liberating calcium from bone, raising alkaline phosphatase (ALP) levels. As discussed in a previous ‘Interpretation’ by Tinnion and Embleton, high (>900 IU/L) ALP levels combined with a phosphate <1.8 mmol/L can indicate metabolic bone disease.20
Vitamin and trace element status: Serum levels of trace elements (zinc, copper and selenium) and fat-soluble vitamins (A, D and E) may help determine insufficiency where there is poor growth despite adequate macronutrient intake, relevant clinical signs (such as poor wound healing in zinc deficiency) or in infants receiving long term (>28 days) PN.
C—clinical assessment
As most preterm infants appear thin with low muscle bulk and scarce subcutaneous fat, differentiating between babies from a nutritional perspective clinically is not possible. Clinical assessment should be guided by information from anthropometry and biochemistry. Assessment of hydration status using capillary refill, urine output and examination for oedema is useful in determining the volume of fluid available for nutrition, and therefore vital when working out how to optimise intake. Oedema (and its subsequent resolution) will impact on weight and so should be taken into account when assessing growth. Concurrent illnesses that might affect nutritional needs should be considered, such as chronic lung disease, which may increase energy requirements. Nutrient absorption and losses (such as stoma output or nasogastric aspirates) as well as nutrient tolerance (such as hyperlipidaemia or hyperglycaemia) should also be considered here.
D—dietary assessment
This element of nutritional assessment is the most challenging but is essential in understanding the extent of shortfalls in nutrient delivery, providing an opportunity to modify and improve nutritional intake. The key here is to move away from considering the feeds and fluids an infant is receiving in terms of their volume, and instead consider their nutritional value. The nutrients to focus on are energy and protein, although consideration needs to be given to the adequacy of micronutrients, particularly those known to be an issue for preterm infants such as sodium, phosphate and fat soluble vitamins (A, D and E). For proprietary ‘stock’ PN solutions, formula milks and fortifier, manufacturers’ datasheets provide the amounts of energy (in kcal) and protein (in grams) in a given volume of fluid (see table 3 for the nutritional content of common feeds). While breast milk will vary widely between individuals, there are published reference values which can enable an estimate of its nutrient content. It is also worth considering at this stage the availability of breast milk, breastfeeding support and alternative feed options where breast milk is unavailable. While alternative formula feeds may provide higher nutrient content, this is far outweighed by the overall nutritional benefits of breast milk, which should be used wherever possible. For pharmacy manufactured individualised PN, pharmacy support is essential, although such PN is provided with a printout providing information on the energy content (usually in kcal/kg) and nitrogen content (usually in grams of nitrogen per kilogram: this can be converted to protein multiplying by a factor of 6.25). It is also worth noting that carbohydrates (including glucose) and protein both provide energy at approximately 4 kcal/g, while fat provides energy at approximately 10 kcal/g.
Nutritional content per 100 ml of common parenteral and enteral feeds
E—evaluation
This final stage in nutritional assessment is to use the above information to make a judgement on an infant's nutritional status. The Academy of Royal Medical Colleges Intercollegiate Course on Human Nutrition uses the framework of ‘what they are’ (their growth, body size and composition), ‘what they eat’ (their dietary intake) and ‘what they can do’ (their functional activity, including physical activity, metabolic capacity, biochemistry and the impact of disease) to consider an individual's nutritional status, which is a helpful approach.21 It is important to consider adequacy of growth and nutrient intakes, and then make a management plan to address any issues identified.
Clinical questions
Should every newborn admitted to the NICU receive nutritional assessment?
Carrying out a nutritional assessment can be time consuming, and may not be necessary for every patient on the NICU. ‘Nutritional screening’ is a way of identifying patients at the highest nutritional risk and in need of full nutritional assessment, and is currently recommended by ESPGHAN. However, the only NICU specific tool is the ‘Ohio Neonatal Nutritionists Screening Criteria for identifying Hospitalized Infants at Highest Nutritional Risk’.22 This complex tool incorporates nutritional assessment and has not been formally validated, but considers infants with a birth weight <1 kg, those with poor growth (<10 g/kg/day after 2 weeks of age) and infants with necrotising enterocolitis, chronic lung disease or gastrointestinal surgical conditions to be at the highest nutritional risk. In the absence of a validated screening tool, these parameters seem a reasonable basis on which to direct nutritional assessments. Using a ‘screening’ approach on a weekly basis ensures that all infants are given brief consideration of their growth and nutritional risk, and also allows the use of nutritional care pathways for specific groups of patients.
Does nutritional assessment of at risk neonates in the NICU improve growth outcomes?
While there is no specific evidence that nutritional assessment per se improves outcomes in neonates, there is evidence that improved nutritional support for preterm infants improves growth.5 Nutritional assessment is essential to properly direct such increased support, such as dietetic input or revision of PN and feeds to address shortfalls in intake.
While a preterm infant is being managed on ‘full’ PN and not yet started on feeds, is nutritional assessment strictly necessary during this period?
Infants managed on ‘full’ PN rarely receive all the nutrition that is prescribed due to concurrent infusions or increasing feeds limiting the amount of fluid available for PN. Furthermore, even when delivered as prescribed, PN is not always ‘total’ and may not meet the full nutritional needs of preterm infants due to restrictions required to maintain stability or energy to protein ratios. Nutritional assessment is therefore important to identify such issues.
In a preterm infant who is fully fed and growing well, is there any place for nutritional assessment over measuring and plotting growth regularly?
While on the surface nutritional assessment seems excessive for an infant in this position, it still has something to add to their ongoing management. Full nutritional assessment in ‘at risk’ infants may reveal reasonable growth growing despite relatively inadequate nutrition. Given that recommendations for nutrient intakes are not secure, it may be that this apparently poor intake is sufficient for their individual needs. Alternatively, it may be that they are not yet showing signs of growth failure, and so require closer monitoring to ensure their growth remains acceptable. Conversely, nutritional assessment may reveal excessive nutrient intakes which need to be addressed. In addition, ‘good’ growth may transpire to be fluid retention or excessive weight gain (measurement of length can be useful here). Once an infant is fully fed, nutritional assessment can also help identify specific issues such as abnormal liver function tests as a result of previous prolonged PN or abnormal markers of bone status.
A nutritional assessment of a neonate demonstrates both poor growth and poor intake. How can this information be used to formulate a management plan?
This is a common situation in preterm infants and can often be relatively simple to deal with by addressing shortfalls in nutrient intake. Selected strategies to address poor nutrient intakes are shown in table 4.
In a neonate with poor growth, does a ‘normal’ nutritional assessment rule out dietary deficiencies as a cause of poor growth?
A ‘normal’ basic nutritional assessment does not exclude dietary deficiencies, especially in an infant who is not growing well. Thought is required as to why seemingly adequate intake is not allowing sufficient growth. It is important to consider whether the presumed intake is being achieved, or whether there is an overestimate of intake due to fluid restriction, failure to take adequate feed volumes (often an issue when infants are ‘demand’ feeding) or excessive losses as vomiting or diarrhoea. Concurrent morbidities, which may increase energy expenditure, or underlying causes for poor growth (such as genetic, metabolic or endocrine conditions) should also be ruled out where appropriate. Detailed attention to biochemistry including assessment of vitamin and trace element status can be helpful, as specific nutrient deficiencies (such as zinc) may be a cause of poor growth. Assessment of acid–base status can be helpful, and sodium status (including urinary sodium) should also be considered, as chronic sodium insufficiency can also lead to poor growth.
Topics for future research
It is still not clear what constitutes optimal growth for preterm infants, and an appropriate growth standard needs to be determined for this group. Such a standard would need to be based on the patterns of growth which lead to optimal neurodevelopmental and metabolic outcomes in later life. As such, there is also a need to establish improved methods of assessing growth on the NICU, particularly with regard to body composition. As discussed above, recommendations for nutrient intakes for preterm infants are also an area where more research is needed, and better understanding of optimal growth in preterm infants will allow more robust recommendations for their optimum intake of key nutrients at critical periods during early life.
Strategies to consider when addressing poor nutrient intakes and growth in neonates
Quiz: True or False?
Current UK reference standards (in the UK-WHO Neonatal and Infant Close Monitoring growth chart) for the growth of preterm infants are based on longitudinal growth data
Restricting the glucose content of PN in response to hyperglycaemia is likely to lead to a reduction in its protein content
Preterm infants have higher nutritional requirements than those born at term
The protein content of PN can be calculated by multiplying its nitrogen content by 6.25
A serum urea less than 1.6 mmol/L suggests inadequate protein intake
A urinary sodium of greater than 10 mmol/L in a preterm infant should be considered abnormally high
Serum sodium is a good marker of total body sodium
Answers are on page 154
Answers to the quiz on page 153
False–the current preterm section of current growth charts is based on cross sectional birth weight data.
TrueIn–order to maintain an energy:protein ratio of around 25k cal/g protein, reducing the glucose (and therefore calorie) content of PN will necessitate a reduction in its protein content. Providing protein in the absence of sufficient energy means that it cannot be used to accrete lean tissue and must be excreted, placing additional metabolic demands on the infant.
True–see Table 2.
True
True
False–it can be normal for preterm infants to have urinary sodium up to 40 mmol/L.
False–Serum sodium is a poor marker of body stores due to tightly regulated homeostatic mechanisms that ensure serum sodium is marinated despite fluctuating intakes and losses.
Clinical bottom line
Nutritional assessment is something that can and should be done easily as part of routine care on NICU, perhaps on a weekly basis.
Basic anthropometry, biochemistry and clinical assessment, together with a dietary assessment can identify suboptimal nutrition and growth at the bedside, allowing interventions to be made that will improve growth and potentially longer term outcomes.
References
Footnotes
Contributors MJJ was responsible for drafting the article and revising it critically for important intellectual content, and approved the final manuscript as submitted. FP, AEW, RMB and AAL revised the article critically for important intellectual content and approved the final manuscript as submitted.
Funding MJJ is a doctoral research fellow supported by the NIHR. AAL's salary is supported by funding from the NIHR Southampton Biomedical Research Centre. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Competing interests RMB has received sponsorship from Mead Johnson, Nestle and Nutricia in lieu of medical advisory work, invited lectures and conference attendance.
Provenance and peer review Commissioned; externally peer reviewed.